MMP8 Is Increased in Lesions and Blood of Acne Inversa Patients: A Potential Link to Skin Destruction and Metabolic Alterations
- PMID: 27843200
- PMCID: PMC5097814
- DOI: 10.1155/2016/4097574
MMP8 Is Increased in Lesions and Blood of Acne Inversa Patients: A Potential Link to Skin Destruction and Metabolic Alterations
Abstract
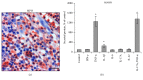
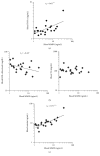
Acne inversa (AI; also designated as hidradenitis suppurativa) is a chronic inflammatory disease with still unknown pathogenesis that affects the intertriginous skin of perianal, inguinal, and axillary sites. It leads to painful nodules, abscesses, and fistulas with malodorous secretion and is frequently associated with metabolic alterations. Here, we demonstrate that one of the most highly upregulated molecules in AI lesions is matrix metalloproteinase 8 (MMP8), an enzyme specialized in the degradation of extracellular matrix components and the HDL component apolipoprotein A-I. Granulocytes, which were present in AI lesions, secreted high amounts of MMP8 especially after TNF-α stimulation. Furthermore, activated fibroblasts but not keratinocytes were found to express MMP8. The high lesional MMP8 levels were accompanied by elevated blood levels that positively correlated with TNF-α blood levels and disease severity assessed by Sartorius score, especially with the number of regions with inflammatory nodules/abscesses and fistulas. Additionally, we found a negative correlation between blood MMP8 and HDL-cholesterol levels, suggesting a contributory role of MMP8 in metabolic alterations in AI. In summary, we demonstrate elevated MMP8 levels in AI lesions, suggest their role in skin destruction and metabolic alterations, and recommend the use of MMP8 as blood biomarker for AI disease activity assessment.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

