Minimal adverse effects profile following implantation of periauricular percutaneous electrical nerve field stimulators: a retrospective cohort study
- PMID: 27843360
- PMCID: PMC5098779
- DOI: 10.2147/MDER.S107426
Minimal adverse effects profile following implantation of periauricular percutaneous electrical nerve field stimulators: a retrospective cohort study
Abstract
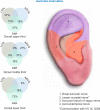
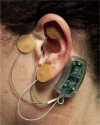
The periauricular percutaneous implantation of the Neuro-Stim System™ family of devices EAD, MFS, and BRIDGE is a procedure involving the use of a non-opiate, neuromodulation analgesic for relieving acute and chronic pain. It has been approved as a minimal-risk procedure by multiple governmental and institutional facilities. This retrospective report of findings will help quantify the incidence of clinically observed bleeding, localized dermatitis, and infections at the implantation sites of the electrode/needle arrays, dermatitis at the site of the generator, and patient syncope. A total of 1,207 devices, each producing up to 16 percutaneous punctures, for a total of 19,312 punctures were monitored for adverse effects, based on retrospective chart audits conducted at six clinical facilities over a 1-year period.
Keywords: Bridge; EAD; MFS; Neuro-Stim System™ devices; adverse effects; clinical risks and discomfort; neuromodulation; percutaneous auricular neurostimulation.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
-
- George MS, Nahas Z, Borckardt JJ, et al. Vagus nerve stimulation for the treatment of depression and other neuropsychiatric disorders. Exp Rev Neurother. 2007;7(1):1–12. - PubMed
-
- Ghoname EA, Craig WF, White PF, et al. Percutaneous electrical nerve stimulation for low back pain. JAMA. 1999;281(9):818–823. - PubMed
-
- Goadsby PJ, Cohen AS, Matharu MS. Trigeminal autonomic cephalgias: diagnosis and treatment. Curr Neurol Neurosci Rep. 2007;7(2):117–125. - PubMed
-
- Zhang Y, Popovic ZB, Bibevesky S, et al. Chronic vagus stimulation improves autonomic control and attenuates systemic inflammation and heart failure progression in a canine high-rate pacing model. Circ Heart Fail. 2009;2(6):692–699. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

