Differences in host breed and diet influence colonization by Campylobacter jejuni and induction of local immune responses in chicken
- PMID: 27843492
- PMCID: PMC5105272
- DOI: 10.1186/s13099-016-0133-1
Differences in host breed and diet influence colonization by Campylobacter jejuni and induction of local immune responses in chicken
Abstract
Background: Chickens are regarded as the main reservoir for human campylobacteriosis. Little is known about the interaction between Campylobacter jejuni (C. jejuni) and chickens. This interaction may be influenced by the stage of maturation of the immune system, developing gut microbiota composition and other factors including breed and diet. Our aim was to investigate the impact of breed, and diet on C. jejuni colonization and host immune responses in chickens. Birds were inoculated with 104 colony forming units (CFU) of C. jejuni or diluent at one (Exp. 1) or 22 (Exp. 2) days post hatch. We compared local immune cell subpopulations, cytokine expression levels, and gut microbiota composition between broiler-type (BT) and layer-type (LT) birds fed with either commercial broiler feed (bf) or layer feed (lf).
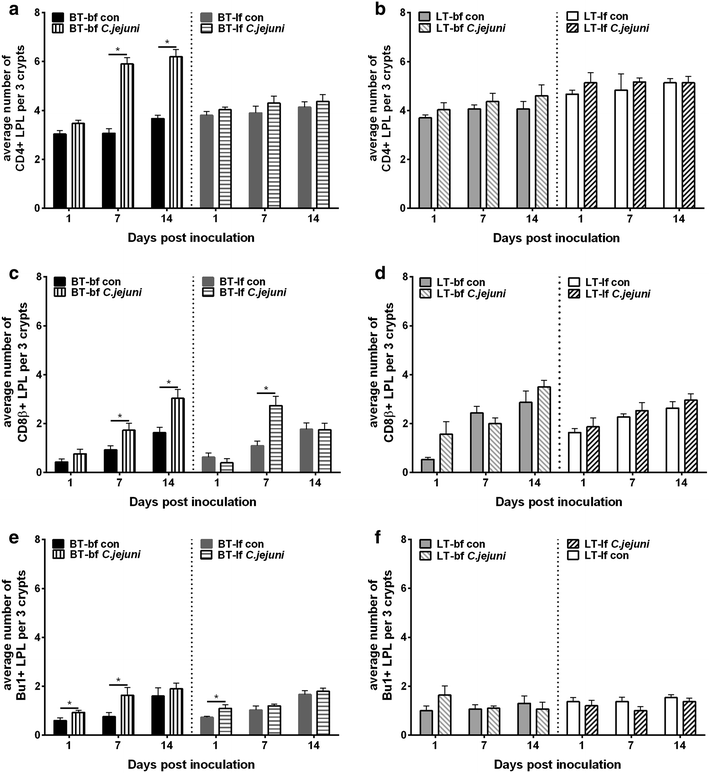
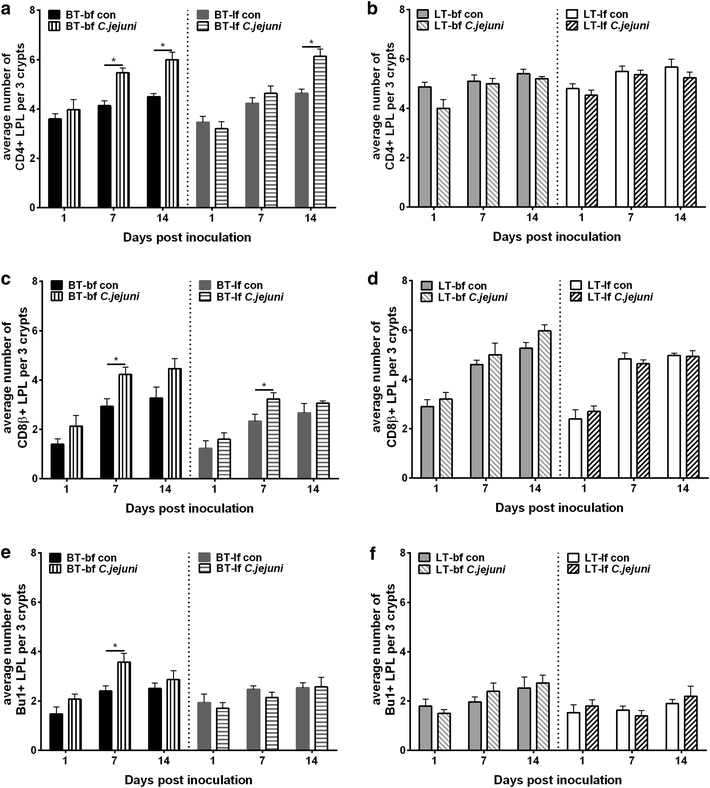
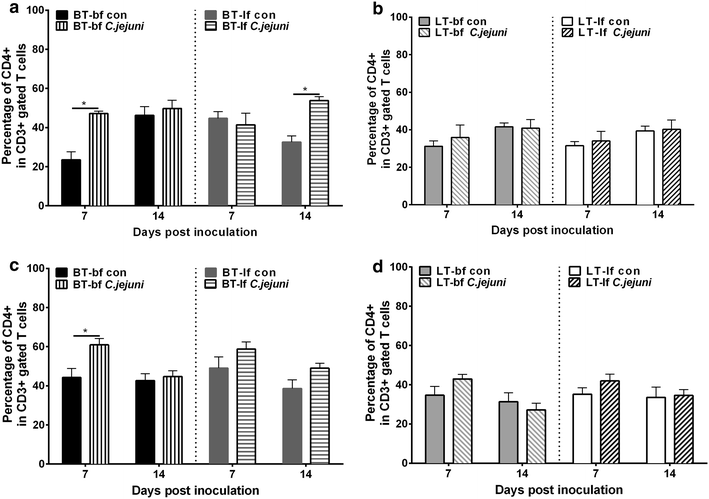
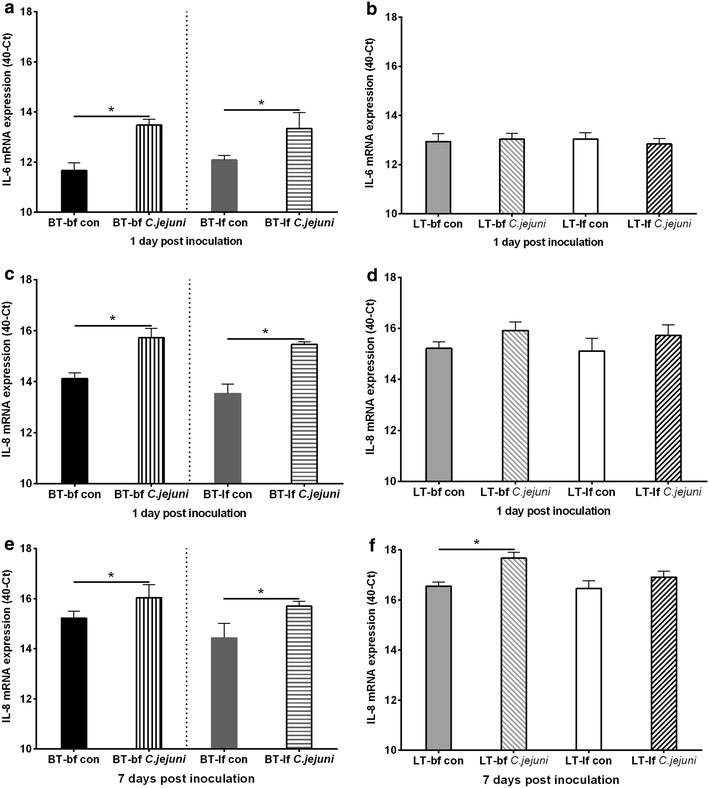
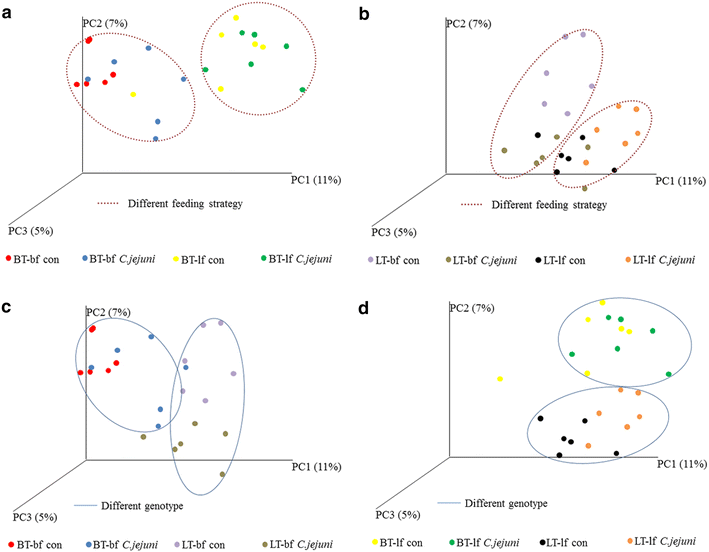
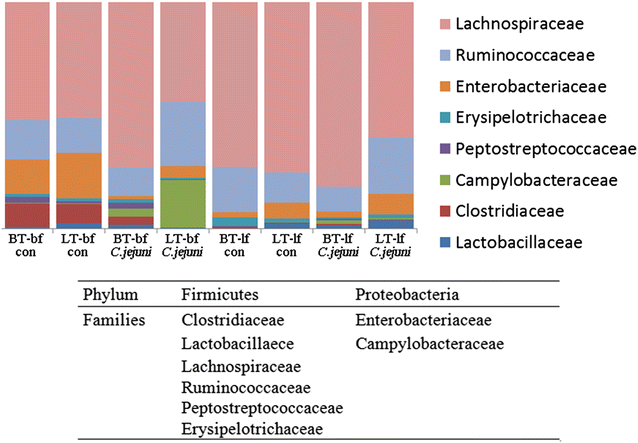
Results: Lower colonization rates were observed in the older age group independent of breed and diet. Independent of breed, birds fed with bf showed higher CFU of C. jejuni compared to lf-fed groups. Campylobacter jejuni-inoculation had a significant effect on lymphocyte numbers and cytokine expression levels in BT birds independent of feeding strategy (p < 0.05). These effects were not detected in LT birds, only LT birds fed with bf showed a significant increase in IL-8-expression at 7 days post C. jejuni inoculation compared to LT-control birds (p < 0.05). Diet influenced gut microbiota composition in a comparable manner between BT and LT birds, but changes in microbiota composition associated with C. jejuni inoculation varied between breeds.
Conclusions: Diet and breed influenced C. jejuni colonization, immune responses and microbiota composition to a different extent comparing between LT and BT birds. The mechanisms behind these differences have to be elucidated further. Our results suggest that selection for more resistant breeds in combination with adapted feeding strategies may help to reduce Campylobacter colonization levels in commercial poultry in the future.
Keywords: Breed; Campylobacter jejuni; Diet; Gut microbiota; Immune response.
Figures
Similar articles
-
Influence of the Gut Microbiota Composition on Campylobacter jejuni Colonization in Chickens.Infect Immun. 2017 Oct 18;85(11):e00380-17. doi: 10.1128/IAI.00380-17. Print 2017 Nov. Infect Immun. 2017. PMID: 28808158 Free PMC article.
-
The influence of age on Campylobacter jejuni infection in chicken.Dev Comp Immunol. 2016 Sep;62:58-71. doi: 10.1016/j.dci.2016.04.020. Epub 2016 Apr 27. Dev Comp Immunol. 2016. PMID: 27131855
-
Infectious bursal disease virus inoculation infection modifies Campylobacter jejuni-host interaction in broilers.Gut Pathog. 2018 Mar 30;10:13. doi: 10.1186/s13099-018-0241-1. eCollection 2018. Gut Pathog. 2018. PMID: 29610580 Free PMC article.
-
Re-thinking the chicken-Campylobacter jejuni interaction: a review.Avian Pathol. 2018 Aug;47(4):352-363. doi: 10.1080/03079457.2018.1475724. Epub 2018 Jun 11. Avian Pathol. 2018. PMID: 29764197 Review.
-
A tolerogenic mucosal immune response leads to persistent Campylobacter jejuni colonization in the chicken gut.Crit Rev Microbiol. 2012 Feb;38(1):17-29. doi: 10.3109/1040841X.2011.615298. Epub 2011 Oct 13. Crit Rev Microbiol. 2012. PMID: 21995731 Review.
Cited by
-
A carvacrol-based product reduces Campylobacter jejuni load and alters microbiota composition in the caeca of chickens.J Appl Microbiol. 2022 Jun;132(6):4501-4516. doi: 10.1111/jam.15521. Epub 2022 Mar 21. J Appl Microbiol. 2022. PMID: 35278017 Free PMC article.
-
Human Immunity Against Campylobacter Infection.Immune Netw. 2019 Dec 2;19(6):e38. doi: 10.4110/in.2019.19.e38. eCollection 2019 Dec. Immune Netw. 2019. PMID: 31921468 Free PMC article. Review.
-
Multi-Omics Reveals Different Strategies in the Immune and Metabolic Systems of High-Yielding Strains of Laying Hens.Front Genet. 2022 Apr 1;13:858232. doi: 10.3389/fgene.2022.858232. eCollection 2022. Front Genet. 2022. PMID: 35432452 Free PMC article.
-
Effects of encapsulated cinnamaldehyde and citral on the performance and cecal microbiota of broilers vaccinated or not vaccinated against coccidiosis.Poult Sci. 2020 Feb;99(2):936-948. doi: 10.1016/j.psj.2019.10.036. Epub 2019 Dec 4. Poult Sci. 2020. PMID: 32029170 Free PMC article.
-
In vitro investigations on interference of selected probiotic candidates with Campylobacter jejuni adhesion and invasion of primary chicken derived cecal and Caco-2 cells.Gut Pathog. 2024 Jun 21;16(1):30. doi: 10.1186/s13099-024-00623-x. Gut Pathog. 2024. PMID: 38907359 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

