Review: Cav2.3 R-type Voltage-Gated Ca2+ Channels - Functional Implications in Convulsive and Non-convulsive Seizure Activity
- PMID: 27843503
- PMCID: PMC5080872
- DOI: 10.2174/1874205X01610010099
Review: Cav2.3 R-type Voltage-Gated Ca2+ Channels - Functional Implications in Convulsive and Non-convulsive Seizure Activity
Abstract
Background: Researchers have gained substantial insight into mechanisms of synaptic transmission, hyperexcitability, excitotoxicity and neurodegeneration within the last decades. Voltage-gated Ca2+ channels are of central relevance in these processes. In particular, they are key elements in the etiopathogenesis of numerous seizure types and epilepsies. Earlier studies predominantly targeted on Cav2.1 P/Q-type and Cav3.2 T-type Ca2+ channels relevant for absence epileptogenesis. Recent findings bring other channels entities more into focus such as the Cav2.3 R-type Ca2+ channel which exhibits an intriguing role in ictogenesis and seizure propagation. Cav2.3 R-type voltage gated Ca2+ channels (VGCC) emerged to be important factors in the pathogenesis of absence epilepsy, human juvenile myoclonic epilepsy (JME), and cellular epileptiform activity, e.g. in CA1 neurons. They also serve as potential target for various antiepileptic drugs, such as lamotrigine and topiramate.
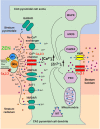
Objective: This review provides a summary of structure, function and pharmacology of VGCCs and their fundamental role in cellular Ca2+ homeostasis. We elaborate the unique modulatory properties of Cav2.3 R-type Ca2+ channels and point to recent findings in the proictogenic and proneuroapoptotic role of Cav2.3 R-type VGCCs in generalized convulsive tonic-clonic and complex-partial hippocampal seizures and its role in non-convulsive absence like seizure activity.
Conclusion: Development of novel Cav2.3 specific modulators can be effective in the pharmacological treatment of epilepsies and other neurological disorders.
Keywords: Absence epilepsy; Afterdepolarisation; Ictal discharges; Low-threshold Ca2+ spike; Plateau potentials; R-type; Seizure.
Figures

Similar articles
-
Altered seizure susceptibility in mice lacking the Ca(v)2.3 E-type Ca2+ channel.Epilepsia. 2006 May;47(5):839-50. doi: 10.1111/j.1528-1167.2006.00541.x. Epilepsia. 2006. PMID: 16686648
-
Hippocampal seizure resistance and reduced neuronal excitotoxicity in mice lacking the Cav2.3 E/R-type voltage-gated calcium channel.J Neurophysiol. 2007 May;97(5):3660-9. doi: 10.1152/jn.01193.2006. Epub 2007 Mar 21. J Neurophysiol. 2007. PMID: 17376845
-
The CaV2.3 R-type voltage-gated Ca2+ channel in mouse sleep architecture.Sleep. 2014 May 1;37(5):881-92. doi: 10.5665/sleep.3652. Sleep. 2014. PMID: 24790266 Free PMC article.
-
The Ca(v)2.3 voltage-gated calcium channel in epileptogenesis--shedding new light on an enigmatic channel.Neurosci Biobehav Rev. 2006;30(8):1122-44. doi: 10.1016/j.neubiorev.2006.07.004. Epub 2006 Sep 11. Neurosci Biobehav Rev. 2006. PMID: 16963121 Review.
-
High-Voltage-Activated Calcium Channels in Epilepsy: Lessons from Humans and Rodents.In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 46. In: Noebels JL, Avoli M, Rogawski MA, Vezzani A, Delgado-Escueta AV, editors. Jasper's Basic Mechanisms of the Epilepsies. 5th edition. New York: Oxford University Press; 2024. Chapter 46. PMID: 39637109 Free Books & Documents. Review.
Cited by
-
Intercommunication between Voltage-Gated Calcium Channels and Estrogen Receptor/Estrogen Signaling: Insights into Physiological and Pathological Conditions.Cells. 2022 Nov 30;11(23):3850. doi: 10.3390/cells11233850. Cells. 2022. PMID: 36497108 Free PMC article. Review.
-
Unconjugated bilirubin modulates neuronal signaling only in wild-type mice, but not after ablation of the R-type/Cav 2.3 voltage-gated calcium channel.CNS Neurosci Ther. 2018 Mar;24(3):222-230. doi: 10.1111/cns.12791. Epub 2017 Dec 23. CNS Neurosci Ther. 2018. PMID: 29274300 Free PMC article.
-
Potential roles of voltage-gated ion channel disruption in Tuberous Sclerosis Complex.Front Mol Neurosci. 2024 Aug 26;17:1404884. doi: 10.3389/fnmol.2024.1404884. eCollection 2024. Front Mol Neurosci. 2024. PMID: 39253727 Free PMC article.
-
Molecular insights into the gating mechanisms of voltage-gated calcium channel CaV2.3.Nat Commun. 2023 Jan 31;14(1):516. doi: 10.1038/s41467-023-36260-2. Nat Commun. 2023. PMID: 36720859 Free PMC article.
-
Epilepsy-linked kinase CDKL5 phosphorylates voltage-gated calcium channel Cav2.3, altering inactivation kinetics and neuronal excitability.Nat Commun. 2023 Dec 11;14(1):7830. doi: 10.1038/s41467-023-43475-w. Nat Commun. 2023. PMID: 38081835 Free PMC article.
References
-
- Bers D.M. Calcium and cardiac rhythms: physiological and pathophysiological. Circ. Res. 2002;90(1):14–17. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
