Antioxidant nutrition in Atlantic salmon (Salmo salar) parr and post-smolt, fed diets with high inclusion of plant ingredients and graded levels of micronutrients and selected amino acids
- PMID: 27843721
- PMCID: PMC5103829
- DOI: 10.7717/peerj.2688
Antioxidant nutrition in Atlantic salmon (Salmo salar) parr and post-smolt, fed diets with high inclusion of plant ingredients and graded levels of micronutrients and selected amino acids
Abstract
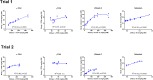
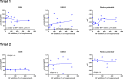
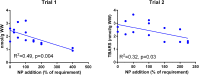
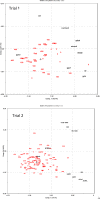
The shift from marine to plant-based ingredients in fish feeds affects the dietary concentrations and bioavailability of micronutrients, amino acids and lipids and consequently warrants a re-evaluation of dietary nutrient recommendations. In the present study, an Atlantic salmon diet high in plant ingredients was supplemented with graded levels of nutrient premix (NP), containing selected amino acids, taurine, cholesterol, vitamins and minerals. This article presents the results on the antioxidant nutrients vitamin C, E and selenium (Se), and effects on tissue redox status. The feed ingredients appeared to contain sufficient levels of vitamin E and Se to cover the requirements to prevent clinical deficiency symptoms. The body levels of α-tocopherol (TOH) in parr and that of Se in parr and post-smolt showed a linear relationship with dietary concentration, while α-TOH in post-smolt seemed to be saturable with a breakpoint near 140 mg kg-1. Ascorbic acid (Asc) concentration in the basal feed was below the expected minimum requirement, but the experimental period was probably too short for the fish to develop visible deficiency symptoms. Asc was saturable in both parr and post-smolt whole body at dietary concentrations of 190 and 63-89 mg kg-1, respectively. Maximum whole body Asc concentration was approximately 40 mg kg-1 in parr and 14 mg kg-1 in post-smolt. Retention ranged from 41 to 10% in parr and from -206 to 12% in post-smolt with increasing NP supplementation. This indicates that the post-smolts had an extraordinarily high consumption of Asc. Analyses of glutathione (GSH) and glutathione disulphide (GSSG) concentrations and the calculated GSH based redox potentials in liver and muscle tissue, indicated only minor effects of diets on redox regulation. However, the post-smolt were more oxidized than the parr. This was supported by the high consumption of Asc and high expression of gpx1 and gpx3 in liver. Based on the present trials, the recommendations for supplementation of vitamin C and E in diets for Atlantic salmon are similar to current practices, e.g. 150 mg kg-1 of α-TOH and 190 mg kg-1 Asc which was the saturating concentration in parr. Higher concentrations than what would prevent clinical deficiency symptoms are necessary to protect fish against incidents of oxidative stress and to improve immune and stress responses. There were no indications that the Se requirement exceeded the current recommendation of 0.3 mg kg-1.
Keywords: Antioxidant nutrients; Atlantic salmon; Nutrient requirement; Redox regulation; Selenium; Vitamin C; Vitamin E.
Conflict of interest statement
Kristin Hamre is an Academic Editor for PeerJ. Joana Silva is employed by Biomar AS, Trondheim, Norway. Bente Torstensen is employed by Marine Harvest ASA, Bergen Norway. Johan Johansen is employed by GIFAS AS, Indyr, Norway. Otherwise there are no competing interests.
Figures


Similar articles
-
Recommendations for dietary level of micro-minerals and vitamin D3 to Atlantic salmon (Salmo salar) parr and post-smolt when fed low fish meal diets.PeerJ. 2019 May 31;7:e6996. doi: 10.7717/peerj.6996. eCollection 2019. PeerJ. 2019. PMID: 31183254 Free PMC article.
-
Atlantic salmon (Salmo salar) require increased dietary levels of B-vitamins when fed diets with high inclusion of plant based ingredients.PeerJ. 2016 Sep 29;4:e2493. doi: 10.7717/peerj.2493. eCollection 2016. PeerJ. 2016. PMID: 27703849 Free PMC article.
-
The effect of micronutrient supplementation on growth and hepatic metabolism in diploid and triploid Atlantic salmon (Salmo salar) parr fed a low marine ingredient diet.Comp Biochem Physiol B Biochem Mol Biol. 2019 Jan;227:106-121. doi: 10.1016/j.cbpb.2018.10.004. Epub 2018 Oct 24. Comp Biochem Physiol B Biochem Mol Biol. 2019. PMID: 30367964
-
A critical life stage of the Atlantic salmon Salmo salar: behaviour and survival during the smolt and initial post-smolt migration.J Fish Biol. 2012 Jul;81(2):500-42. doi: 10.1111/j.1095-8649.2012.03370.x. J Fish Biol. 2012. PMID: 22803722 Review.
-
Methionine: An Indispensable Amino Acid in Cellular Metabolism and Health of Atlantic Salmon.Aquac Nutr. 2023 Oct 27;2023:5706177. doi: 10.1155/2023/5706177. eCollection 2023. Aquac Nutr. 2023. PMID: 37927379 Free PMC article. Review.
Cited by
-
Mesopelagic Species and Their Potential Contribution to Food and Feed Security-A Case Study from Norway.Foods. 2020 Mar 16;9(3):344. doi: 10.3390/foods9030344. Foods. 2020. PMID: 32188085 Free PMC article.
-
Early nutritional intervention can improve utilisation of vegetable-based diets in diploid and triploid Atlantic salmon (Salmo salar L.).Br J Nutr. 2017 Jul;118(1):17-29. doi: 10.1017/S0007114517001842. Epub 2017 Jul 24. Br J Nutr. 2017. PMID: 28735572 Free PMC article.
-
One-carbon metabolism nutrients impact the interplay between DNA methylation and gene expression in liver, enhancing protein synthesis in Atlantic salmon.Epigenetics. 2024 Dec;19(1):2318517. doi: 10.1080/15592294.2024.2318517. Epub 2024 Feb 25. Epigenetics. 2024. PMID: 38404006 Free PMC article.
-
Somatotropic Axis Regulation Unravels the Differential Effects of Nutritional and Environmental Factors in Growth Performance of Marine Farmed Fishes.Front Endocrinol (Lausanne). 2018 Nov 27;9:687. doi: 10.3389/fendo.2018.00687. eCollection 2018. Front Endocrinol (Lausanne). 2018. PMID: 30538673 Free PMC article. Review.
-
Effectiveness of functional ingredients to enhance gill disease in Atlantic salmon (Salmo salar, L.).PLoS One. 2024 Jun 20;19(6):e0304112. doi: 10.1371/journal.pone.0304112. eCollection 2024. PLoS One. 2024. PMID: 38900829 Free PMC article.
References
-
- Alfthan G, Eurola M, Ekholm P, Venäläinen E-R, Root T, Korkalainen K, Hartikainen H, Salminen P, Hietaniemi V, Aspila P, Aro A, Selenium Working Group Effects of nationwide addition of selenium to fertilizers on foods, and animal and human health in Finland: from deficiency to optimal selenium status of the population. Journal of Trace Elements in Medicine and Biology. 2015;31:142–147. doi: 10.1016/j.jtemb.2014.04.009. - DOI - PubMed
-
- Baker RTM, Davies SJ. Modulation of tissue alpha-tocopherol in African catfish, Clarias gariepinus (Burchell), fed oxidized oils, and the compensatory effect of supplemental dietary vitamin E. Aquaculture Nutrition. 1997;3(2):91–97. doi: 10.1046/j.1365-2095.1997.00078.x. - DOI
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

