Randomized Phase II Study of Duligotuzumab (MEHD7945A) vs. Cetuximab in Squamous Cell Carcinoma of the Head and Neck (MEHGAN Study)
- PMID: 27843803
- PMCID: PMC5086582
- DOI: 10.3389/fonc.2016.00232
Randomized Phase II Study of Duligotuzumab (MEHD7945A) vs. Cetuximab in Squamous Cell Carcinoma of the Head and Neck (MEHGAN Study)
Abstract
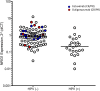
Background: Duligotuzumab, a novel dual-action humanized IgG1 antibody that blocks ligand binding to epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 3 (HER3), inhibits signaling from all ligand-dependent HER dimers, and can elicit antibody-dependent cell-mediated cytotoxicity. High tumor-expression of neuregulin 1 (NRG1), a ligand to HER3, may enhance sensitivity to duligotuzumab.
Methods: This multicenter, open-label, randomized phase II study (MEHGAN) evaluated drug efficacy in patients with recurrent/metastatic (R/M) squamous cell carcinoma of the head and neck (SCCHN) progressive on/after chemotherapy and among patients with NRG1-high tumors. Patients received duligotuzumab (1100 mg IV, q2w) or cetuximab (400 mg/m2 load, 250 mg/m2 IV, q1w) until progression or intolerable toxicity. Tumor samples were assayed for biomarkers [NRG1, ERBB3, and human papillomavirus (HPV) status].
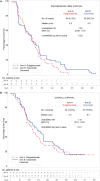
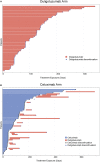
Results: Patients (N = 121) were randomized (duligotuzumab:cetuximab; 59:62), median age 62 years; ECOG 0-2. Both arms (duligotuzumab vs. cetuximab, respectively) showed comparable progression-free survival [4.2 vs. 4.0 months; HR: 1.23 (90% confidence interval (CI): 0.89-1.70)], overall survival [7.2 vs. 8.7 months; HR 1.15 (90% CI: 0.81-1.63)], and objective response rate (12 vs. 14.5%), with no difference between patients with NRG1-high tumors or ERBB3-low tumors. Responses in both arms were confined to HPV-negative patients. Grade ≥3 adverse events (AEs) (duligotuzumab vs. cetuximab, respectively) included infections (22 vs. 11.5%) and GI disorders (17 vs. 7%), contributing to higher rates of serious AEs (41 vs. 29.5%). Metabolic disorders were less frequent with duligotuzumab (10 vs. 16%); any grade rash-related events were less with duligotuzumab (49 vs. 67%).
Conclusion: While several lines of preclinical evidence had supported the premise that the blockade of HER3 in addition to that of EGFR may improve outcomes for patients with R/M SCCHN overall or specifically in those patients whose tumors express high levels of NRG1, this study provided definitive clinical evidence refuting this hypothesis. Duligotuzumab did not improve patient outcomes in comparison to cetuximab despite frequent expression of NRG1. These data indicate that inhibition of EGFR alone is sufficient to block EGFR-HER3 signaling, suggesting that HER2 plays a minimal role in this disease. Extensive biomarker analyses further show that HPV-negative SCCHN but not HPV-positive SCCHN are most likely to respond to EGFR blockage by cetuximab or duligotuzumab.
Keywords: EGFR; HER3; HPV; MEHD7945A; NRG1; SCCHN; cetuximab; duligotuzumab.
Figures


Comment in
-
Commentary: Randomized Phase II Study of Duligotuzumab (MEHD7945A) vs. Cetuximab in Squamous Cell Carcinoma of the Head and Neck (MEHGAN Study).Front Oncol. 2017 Mar 13;7:31. doi: 10.3389/fonc.2017.00031. eCollection 2017. Front Oncol. 2017. PMID: 28348975 Free PMC article. No abstract available.
References
-
- Vermorken JB, Trigo J, Hitt R, Koralewski P, Diaz-Rubio E, Rolland F, et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J Clin Oncol (2007) 25(16):2171–7.10.1200/jco.2006.06.7447 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

