A theoretical study of benzaldehyde derivatives as tyrosinase inhibitors using Ab initio calculated NQCC parameters
- PMID: 27844007
- PMCID: PMC5019207
A theoretical study of benzaldehyde derivatives as tyrosinase inhibitors using Ab initio calculated NQCC parameters
Abstract
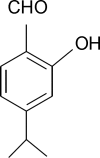
Tyrosinase is a multifunctional copper-containing enzyme. It can catalyze two distinct reactions of melanin synthesis and benzaldehyde derivatives, which are potential tyrosinase inhibitors. To find the relationships between charge distributions of benzaldehyde and their pharmaceutical behavior, the present study aimed at investigating nuclear quadrupole coupling constants of quadrupolare nuclei in the functional benzaldehyde group and calculating some its derivatives. In addition, the differences between the electronic structures of various derivatives of this depigmenting drug were examined. All ab initio calculations were carried out using Gaussian 03. The results predicted benzaldehyde derivatives to be bicentral inhibitors; nevertheless, the oxygen or hydrogen contents of the aldehyde group were not found to be the only active sites. Furthermore with the presence of the aldehyde group, the terminal methoxy group in C4 was found to contribute to tyrosinase inhibitory activities. In addition, an oxygen atom with high charge density in the side chain was found to play an important role in its inhibitory effect.
Keywords: Charge density; Gaussian; NQR; quadrupolar nuclei.
Figures
References
-
- Briganti S, Camera E, Picardo M. Chemical and instrumental approaches to treat hyperpigmentation. Pigment Cell Res. 2003;16:101–108. - PubMed
-
- Sanchez-Ferrer A, Rodriguez-Lopez JN, Garcia-Canovas F, Garcia-Carmona F. Tyrosinase: a comprehensive review of its mechanism. Biochim Biophys Acta. 1995;1247:1–5. - PubMed
-
- Seo SY, Sharma VK, Sharma N. Mushroom tyrosinase: recent prospects. J Agric Food Chem. 2003;51:2837–2853. - PubMed
-
- Maeda K, Fukuda M. In vitro effectiveness of several whitening cosmetic components in human melanocytes. J Soc Cosmet Chem. 1991;42:361–368.
-
- Delogu G, Podda G, Corda M, Fadda MB, Fais A, Era B. Synthesis and biological evaluation of novel series of bis-salicylaldehydes as mushroom tyrosinase inhibitors. Bioorg Med Chem Lett. 2010;20:6138–6140. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous