An iron detection system determines bacterial swarming initiation and biofilm formation
- PMID: 27845335
- PMCID: PMC5109203
- DOI: 10.1038/srep36747
An iron detection system determines bacterial swarming initiation and biofilm formation
Abstract
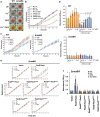
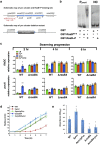
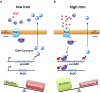
Iron availability affects swarming and biofilm formation in various bacterial species. However, how bacteria sense iron and coordinate swarming and biofilm formation remains unclear. Using Serratia marcescens as a model organism, we identify here a stage-specific iron-regulatory machinery comprising a two-component system (TCS) and the TCS-regulated iron chelator 2-isocyano-6,7-dihydroxycoumarin (ICDH-Coumarin) that directly senses and modulates environmental ferric iron (Fe3+) availability to determine swarming initiation and biofilm formation. We demonstrate that the two-component system RssA-RssB (RssAB) directly senses environmental ferric iron (Fe3+) and transcriptionally modulates biosynthesis of flagella and the iron chelator ICDH-Coumarin whose production requires the pvc cluster. Addition of Fe3+, or loss of ICDH-Coumarin due to pvc deletion results in prolonged RssAB signaling activation, leading to delayed swarming initiation and increased biofilm formation. We further show that ICDH-Coumarin is able to chelate Fe3+ to switch off RssAB signaling, triggering swarming initiation and biofilm reduction. Our findings reveal a novel cellular system that senses iron levels to regulate bacterial surface lifestyle.
Figures




References
-
- Andrews S. C., Robinson A. K. & Rodriguez-Quinones F. Bacterial iron homeostasis. FEMS Microbiol Rev 27, 215–237 (2003). - PubMed
-
- Touati D. Iron and oxidative stress in bacteria. Arch Biochem Biophys 373, 1–6 (2000). - PubMed
-
- Marx J. J. Iron and infection: competition between host and microbes for a precious element. Best Pract Res Clin Haematol 15, 411–426 (2002). - PubMed
-
- Braun V. & Hantke K. Recent insights into iron import by bacteria. Curr Opin Chem Biol 15, 328–334 (2011). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

