Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response
- PMID: 27845343
- PMCID: PMC5116072
- DOI: 10.1038/ncomms13464
Directed transport of neutrophil-derived extracellular vesicles enables platelet-mediated innate immune response
Abstract
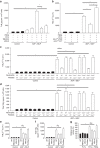
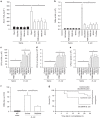
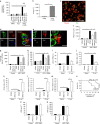
The innate immune response to bacterial infections requires the interaction of neutrophils and platelets. Here, we show that a multistep reciprocal crosstalk exists between these two cell types, ultimately facilitating neutrophil influx into the lung to eliminate infections. Activated platelets adhere to intravascular neutrophils through P-selectin/P-selectin glycoprotein ligand-1 (PSGL-1)-mediated binding, a primary interaction that allows platelets glycoprotein Ibα (GPIbα)-induced generation of neutrophil-derived extracellular vesicles (EV). EV production is directed by exocytosis and allows shuttling of arachidonic acid into platelets. EVs are then specifically internalized into platelets in a Mac1-dependent fashion, and relocated into intracellular compartments enriched in cyclooxygenase1 (Cox1), an enzyme processing arachidonic acid to synthesize thromboxane A2 (TxA2). Finally, platelet-derived-TxA2 elicits a full neutrophil response by inducing the endothelial expression of ICAM-1, intravascular crawling, and extravasation. We conclude that critical substrate-enzyme pairs are compartmentalized in neutrophils and platelets during steady state limiting non-specific inflammation, but bacterial infection triggers regulated EV shuttling resulting in robust inflammation and pathogen clearance.
Figures



References
-
- Ware L. B. & Matthay M. A. The acute respiratory distress syndrome. N. Engl. J. Med. 342, 1334–1349 (2000). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

