Recent Progress in the Molecular Recognition and Therapeutic Importance of Interleukin-1 Receptor-Associated Kinase 4
- PMID: 27845762
- PMCID: PMC6274160
- DOI: 10.3390/molecules21111529
Recent Progress in the Molecular Recognition and Therapeutic Importance of Interleukin-1 Receptor-Associated Kinase 4
Abstract
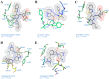
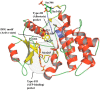
Toll-like receptors (TLRs) are the most upstream pattern recognition receptors in the cell, which detect pathogen associated molecular patterns and initiate signal transduction, culminating in the transcription of pro-inflammatory cytokines and antiviral interferon. Interleukin-1 receptor-associated kinase 4 (IRAK4) is a key mediator in TLR (except for TLR3) and interleukin-1 receptor signaling pathways. The loss of kinase function of IRAK4 is associated with increased susceptibility to various pathogens, while its over-activation causes autoimmune diseases such as rheumatoid arthritis, systemic lupus erythematosus, and cancer. The therapeutic importance of this master kinase has been advocated by a number of recent preclinical studies, where potent inhibitors have been administered to improve various TLR-mediated pathologies. Increasing studies of X-ray crystallographic structures with bound inhibitors have improved our knowledge on the molecular recognition of ligands by IRAK4, which will be crucial for the development of new inhibitors with improved potencies. In this review, we briefly discuss the structural aspect of ligand recognition by IRAK4 and highlight its therapeutic importance in the context of TLR-associated unmet medical needs.
Keywords: IRAK4; TLR; X-ray crystallography; autoimmunity; inhibitor.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
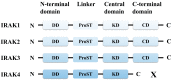
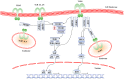
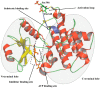
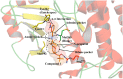
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

