Modified-Release and Conventional Glucocorticoids and Diurnal Androgen Excretion in Congenital Adrenal Hyperplasia
- PMID: 27845856
- PMCID: PMC5470768
- DOI: 10.1210/jc.2016-2855
Modified-Release and Conventional Glucocorticoids and Diurnal Androgen Excretion in Congenital Adrenal Hyperplasia
Abstract
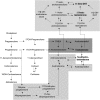
Context: The classic androgen synthesis pathway proceeds via dehydroepiandrosterone, androstenedione, and testosterone to 5α-dihydrotestosterone. However, 5α-dihydrotestosterone synthesis can also be achieved by an alternative pathway originating from 17α-hydroxyprogesterone (17OHP), which accumulates in congenital adrenal hyperplasia (CAH). Similarly, recent work has highlighted androstenedione-derived 11-oxygenated 19-carbon steroids as active androgens, and in CAH, androstenedione is generated directly from 17OHP. The exact contribution of alternative pathway activity to androgen excess in CAH and its response to glucocorticoid (GC) therapy is unknown.
Objective: We sought to quantify classic and alternative pathway-mediated androgen synthesis in CAH, their diurnal variation, and their response to conventional GC therapy and modified-release hydrocortisone.
Methods: We used urinary steroid metabolome profiling by gas chromatography-mass spectrometry for 24-hour steroid excretion analysis, studying the impact of conventional GCs (hydrocortisone, prednisolone, and dexamethasone) in 55 adults with CAH and 60 controls. We studied diurnal variation in steroid excretion by comparing 8-hourly collections (23:00-7:00, 7:00-15:00, and 15:00-23:00) in 16 patients with CAH taking conventional GCs and during 6 months of treatment with modified-release hydrocortisone, Chronocort.
Results: Patients with CAH taking conventional GCs showed low excretion of classic pathway androgen metabolites but excess excretion of the alternative pathway signature metabolites 3α,5α-17-hydroxypregnanolone and 11β-hydroxyandrosterone. Chronocort reduced 17OHP and alternative pathway metabolite excretion to near-normal levels more consistently than other GC preparations.
Conclusions: Alternative pathway-mediated androgen synthesis significantly contributes to androgen excess in CAH. Chronocort therapy appears superior to conventional GC therapy in controlling androgen synthesis via alternative pathways through attenuation of their major substrate, 17OHP.
Figures




References
-
- Han TS, Walker BR, Arlt W, Ross RJ. Treatment and health outcomes in adults with congenital adrenal hyperplasia. Nat Rev Endocrinol. 2013;10(2):115–124. - PubMed
-
- Krone N, Braun A, Roscher AA, Knorr D, Schwarz HP. Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J Clin Endocrinol Metab. 2000;85(3):1059–1065. - PubMed
-
- Auchus RJ. The backdoor pathway to dihydrotestosterone. Trends Endocrinol Metab. 2004;15(9):432–438. - PubMed
-
- Arlt W, Walker EA, Draper N, Ivison HE, Ride JP, Hammer F, Chalder SM, Borucka-Mankiewicz M, Hauffa BP, Malunowicz EM, Stewart PM, Shackleton CH. Congenital adrenal hyperplasia caused by mutant P450 oxidoreductase and human androgen synthesis: analytical study. Lancet. 2004;363(9427):2128–2135. - PubMed
-
- Wilson JD, Auchus RJ, Leihy MW, Guryev OL, Estabrook RW, Osborn SM, Shaw G, Renfree MB. 5alpha-androstane-3alpha,17beta-diol is formed in tammar wallaby pouch young testes by a pathway involving 5alpha-pregnane-3alpha,17alpha-diol-20-one as a key intermediate. Endocrinology. 2003;144(2):575–580. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

