Efficacy and Safety of Oral Beclomethasone Dipropionate in Ulcerative Colitis: A Systematic Review and Meta-Analysis
- PMID: 27846307
- PMCID: PMC5113024
- DOI: 10.1371/journal.pone.0166455
Efficacy and Safety of Oral Beclomethasone Dipropionate in Ulcerative Colitis: A Systematic Review and Meta-Analysis
Abstract
Background and aim: We performed a systematic review and meta-analysis of all the available evidence comparing efficacy and safety of oral prolonged released beclomethasone dipropionate (BDP) to active oral controls in patients with mild-to-moderate ulcerative colitis (UC). A subgroup-analysis compared the effectiveness of BDP and 5-ASA.
Methods: Literature research was performed in different databases, as well as manual search to identify abstracts from international meetings with data not included in extensive publications. Experts in the field and companies involved in BDP development and manufacture were contacted to identify unpublished studies used for registration purposes. Dichotomous data were pooled to obtain odds ratio meta-analysis.
Results: Five randomized controlled trials that compared oral BDP 5mg/day vs. all oral active controls in treating UC were identified as eligible. Efficacy and safety have been addressed after 4-week treatment period. One study evaluated efficacy and safety of BDP vs. prednisone and 4 of BDP vs. 5-ASA. Treatment with oral BDP 5 mg/day induces a significant better clinical response compared to oral 5-ASA (OR 1.86, 95% CI = 1.23-2.82, P = 0.003). The effect is detectable even when the comparison to prednisone is added (OR 1.41, 95% CI = 1.03-1.93, P = 0.03). Data on remission indicate that the potential clinical efficacy of BDP may be better than 5-ASA (OR 1.55, 95% CI = 1.00-2.40, P = 0.05). This difference is lost when the comparison with prednisone is added (OR 1.30, 95% CI = 0.76-2.23, P = 0.34). The safety analysis showed no differences between BDP and 5-ASA (OR 0.55, 95% CI = 0.24-1.27, P = 0.16). The lack of difference is maintained even when the study with prednisone is added (OR 0.67, 95% CI = 0.44-1.01, P = 0.06). However, the trend of difference is clear and indicates a more favourable safety profile of BDP compared to 5-ASA and PD.
Conclusions: Oral prolonged release BDP showed a superior efficacy vs. oral 5-ASA in inducing clinical improvement of mild-to-moderate UC with a similar safety profile.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





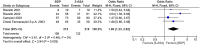


References
-
- Gabbani T, Manetti N, Bagnoli S, Annese V. Beclomethasone dipropionate for the treatment of ulcerative colitis. Expert Opin Orphan D 2015; 3: 87–96
-
- Both H, Torp-Pedersen K, Kreiner S, Hendriksen C, Binder V. Clinical appearance at diagnosis of ulcerative colitis and Crohn’s disease in a regional patient group. Scand J Gastroenterol 1983; 18: 987–991 [] - PubMed
-
- Mulder CJ, Rondas AA, Wiltink EH, Tytgat GN. Topical corticosteroids in inflammatory bowel disease. Neth J Med 1989; 35 (Suppl. 1): S27–34 [] - PubMed
-
- Harting JW. New developments in the pharmacotherapy of inflammatory bowel disease. Pharm Weekbl Sci 1992; 14: 275–86 [] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

