Pharmacological treatment other than corticosteroids, intravenous immunoglobulin and plasma exchange for Guillain-Barré syndrome
- PMID: 27846348
- PMCID: PMC6464710
- DOI: 10.1002/14651858.CD008630.pub4
Pharmacological treatment other than corticosteroids, intravenous immunoglobulin and plasma exchange for Guillain-Barré syndrome
Update in
-
Pharmacological treatment other than corticosteroids, intravenous immunoglobulin and plasma exchange for Guillain-Barré syndrome.Cochrane Database Syst Rev. 2020 Jan 25;1(1):CD008630. doi: 10.1002/14651858.CD008630.pub5. Cochrane Database Syst Rev. 2020. PMID: 31981368 Free PMC article.
Abstract
Background: Plasma exchange and intravenous immunoglobulin, but not corticosteroids, are beneficial in Guillain-Barré syndrome (GBS). The efficacy of other pharmacological agents is unknown. This review was first published in 2011 and updated in 2013 and 2016.
Objectives: To assess the effects of pharmacological agents other than plasma exchange, intravenous immunoglobulin and corticosteroids for GBS.
Search methods: On 18 January 2016, we searched the Cochrane Neuromuscular Specialised Register, Cochrane Central Register of Controlled Trials, MEDLINE, and Embase for treatments for GBS. We also searched clinical trials registries.
Selection criteria: We included all randomised controlled trials (RCTs) or quasi-RCTs of acute GBS (within four weeks from onset) of all types and degrees of severity, and in individuals of all ages. We discarded trials that investigated only corticosteroids, intravenous immunoglobulin or plasma exchange. We included other pharmacological treatments or combinations of treatments compared with no treatment, placebo or another treatment. We also identified a number of non-randomised studies during the search, the results of which we considered in the Discussion.
Data collection and analysis: We followed standard Cochrane methodology.
Main results: We identified no new trials during this update of the review. In previous versions of this review we identified only very low quality evidence for four different interventions published in four studies. Each study had a high risk of bias in at least one respect. One RCT with 19 participants comparing interferon beta-1a and placebo showed no clinically important difference in any outcome between groups. Another with 10 participants comparing brain-derived neurotrophic factor and placebo showed no clinically important difference in any outcome between groups. A third with 37 participants comparing cerebrospinal fluid filtration and plasma exchange also showed no clinically important difference in any outcome between groups. In a fourth with 43 participants, the risk ratio for an improvement by one or more disability grade after eight weeks was greater with the Chinese herbal medicine tripterygium polyglycoside than with corticosteroids (risk ratio 1.47; 95% confidence interval 1.02 to 2.11); other outcomes in this trial showed no difference. Serious adverse events were uncommon with each of these treatments and in the control groups.
Authors' conclusions: The quality of the evidence was very low. Three small RCTs, comparing interferon beta-1a or brain-derived neurotrophic factor with placebo, and cerebrospinal fluid filtration with plasma exchange, showed no significant benefit or harm for any of the interventions. A fourth small trial showed that the Chinese herbal medicine, tripterygium polyglycoside, hastened recovery in people with GBS to a greater extent than corticosteroids, but this result needs confirmation. We were unable to draw any useful conclusions from the few observational studies we identified.
Conflict of interest statement
All three authors have conducted trials which were relevant for inclusion in this review.
JP was involved in the Pritchard 2003 trial in this review, which was funded by Serono.
RACH's department received financial support for two trials included in this review, from Amgen for Bensa 2000 and from Serono for Pritchard 2003. Neither study showed a significant beneficial effect of the drug tested. More than eight years ago, RACH's department also received funding for trials in multiple sclerosis. RACH holds or has held consultancies with Baxter, CSL Behring, Grifols, LFB and Octapharma, all of which manufacture human immunoglobulin used in the treatment of GBS, but specifically excluded from this review. RACH holds or has held consultancies with BMS, Novartis and UCB, which are not known to him to be marketing or developing drugs for use in GBS. RACH serves as honorary board member of the GBS/CIDP Foundation International and medical patron of gain, charities which serve the interests of people with GBS.
RDMH has nothing to declare relating to the current review, but declares financial relationships with Baxter Healthcare Ltd (payment of nurse salary to department); Grifols (development of educational presentations, conference travel expenses); CSL Behring (advisory board membership, conference travel expenses, supply of clinical equipment); and Kent Institute of Medicine and Surgery (shareholder). He is a co‐author of one of the studies included in the review, Bensa 2000.
RB is Managing Editor of Cochrane Neuromuscular. She has no known conflicts of interest. She was not involved in the later stages of the editorial process other than as a review author.
Two review authors (RACH and RDMH) have declared relationships with commercial companies producing IVIg, which is used in the treatment of GBS. IVIg is explicitly excluded from the review and is not considered to be a competing intervention with any of the currently included interventions. Cochrane Neuromuscular is satisfied after consultation with the Funding Arbiter that these relationships do not represent a conflict of interest in this version of the review.
Figures
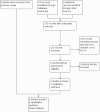















Update of
-
Pharmacological interventions for pruritus in adult palliative care patients.Cochrane Database Syst Rev. 2013 Jun 9;(6):CD008320. doi: 10.1002/14651858.CD008320.pub2. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2016 Nov 15;11:CD008630. doi: 10.1002/14651858.CD008630.pub4. Update in: Cochrane Database Syst Rev. 2016 Nov 16;11:CD008320. doi: 10.1002/14651858.CD008320.pub3. PMID: 23749733 Updated.
References
References to studies included in this review
-
- Bensa S, Hadden RD, Hahn A, Hughes RA, Willison HJ. Randomized controlled trial of brain‐derived neurotrophic factor in Guillain‐Barré syndrome: a pilot study. European Journal of Neurology 2000;7(4):423‐6. [; PUBMED: 10971602] - PubMed
-
2685117
-
- Pritchard J, Gray IA, Hughes RAC, Idrissova ZR, Lecky BRF, Swan AV, et al. A pilot randomised, double‐blind, placebo‐controlled exploratory safety study of the use of interferon‐beta 1a in the treatment of Guillain‐Barré syndrome. Journal of the Peripheral Nervous System 2003;8(Suppl 1):52. []
- Pritchard J, Gray IA, Idrissova ZR, Lecky BR, Sutton IJ, Swan AV, et al. A randomized controlled trial of recombinant interferon‐beta 1a in Guillain‐Barré syndrome. Neurology 2003;61(9):1282‐4. [; PUBMED: 14610140] - PubMed
-
2685119
-
- Wollinsky KH, Hülser PJ, Brinkmeier H, Aulkemeyer P, Bössenecker W, Huber‐Hartmann KH, et al. CSF filtration is an effective treatment of Guillain‐Barré syndrome: a randomized clinical trial. Neurology 2001;57(5):774‐80. [; PUBMED: 11552002] - PubMed
-
2685122
-
- Zhang X, Xia J, Ye H. Effect of tripterygium polyglycoside on interleukin‐6 in patients with Guillain‐Barre syndrome. Zhongguo Zhong Xi Yi Jie He Za Zhi Zhongguo Zhongxiyi Jiehe Zazhi [Chinese Journal of Integrated Traditional & Western Medicine] Zhongguo Zhong Xi Yi Jie He Xue Hui, Zhongguo Zhong Yi Yan Jiu Yuan Zhu Ban 2000;20(5):332‐4. [; PUBMED: 11789240] - PubMed
-
2685124
References to studies excluded from this review
-
- Ahuja GK, Mohandas S, Virani V. Cyclophosphamide in Landry‐Guillain‐Barré syndrome. Acta Neurologica 1980;2(3):186‐90. [; PUBMED: 7415884] - PubMed
-
2685126
-
- Bos Eyssen ME, Doorn PA, Jacobs BC, Steyerberg EW, Voort PH, Zandstra DF, et al. Selective digestive tract decontamination decreases time on ventilator in Guillain‐Barre syndrome. Neurocritical Care 2011;15(1):128‐33. [] - PubMed
-
2685128
-
- Colin‐Jones DG, Heathfield KWG. 6‐mercaptopurine in polyradiculoneuropathy. Lancet 1965;2:739. []
-
2685130
-
- Créange A, Lerat H, Meyrignac C, Degos JD, Gherardi R, Cesaro P. Treatment of Guillain‐Barré syndrome with interferon‐beta. Lancet 1998;352(9125):368‐9. [] - PubMed
-
2685132
-
- Grandis D, Santoro L, Benedetto P. L‐acetylcarnitine in the treatment of patients with peripheral neuropathies: a short term, double‐blind clinical study of 426 patients. Clinical Drug Investigation 1995;10(6):317‐22. [] - PubMed
-
2685134
References to ongoing studies
-
- Davidson AL, Chavada G, Overell JR, Willison HJ. A double blind, randomised controlled phase II trial of complement inhibition in Guillain‐Barré syndrome. Journal of the Peripheral Nervous System 2014;19(3):250‐89. [; DOI: 10.1111/jns.12083; NCT02029378] - DOI
- NCT02029378. Inhibition of complement activation (eculizumab) in Guillain‐Barre syndrome study (ICA‐GBS). clinicaltrials.gov/ct2/show/NCT02029378 (first received 6 January 2014). []
-
2685185
-
- NCT02493725. JET‐GBS ‐ Japanese eculizumab trial for GBS. clinicaltrials.gov/ct2/show/NCT02493725 (first received 7 July 2015). []
-
2685188
Additional references
-
- Alvarez‐Lario B, Prieto‐Tejedo R, Colazo‐Burlato M, Macarron‐Vicente J. Severe Guillain‐Barré syndrome in a patient receiving anti‐TNF therapy. Consequence or coincidence. A case‐based review. Clinical Rheumatology 2013;32(9):1407‐12. - PubMed
-
- Asbury AK, Cornblath DR. Assessment of current diagnostic criteria for Guillain‐Barré syndrome. Annals of Neurology 1990;27(Suppl):S21‐4. [PUBMED: 2194422] - PubMed
-
- Bernsen RA, Jager AE, Schmitz PI, Meché FG. Residual physical outcome and daily living 3 to 6 years after Guillain‐Barré syndrome. Neurology 1999;53(2):409‐10. [PUBMED: 10430437] - PubMed
-
- Brinkmeier H, Wollinsky KH, Hulser PJ, Seewald MJ, Mehrkens H, Kornhuber HH, et al. The acute paralysis in Guillain‐Barré syndrome is related to a Na+ channel blocking factor in the cerebrospinal fluid. Pflugers Archiv: European Journal of Physiology 1992;421:552‐7. [PUBMED: 1331974] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

