A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1-/- mice is correlated to increased cellular senescence
- PMID: 27846439
- PMCID: PMC5109248
- DOI: 10.1016/j.redox.2016.10.014
A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1-/- mice is correlated to increased cellular senescence
Abstract
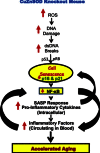
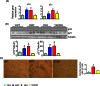
In contrast to other mouse models that are deficient in antioxidant enzymes, mice null for Cu/Zn-superoxide dismutase (Sod1-/- mice) show a major decrease in lifespan and several accelerated aging phenotypes. The goal of this study was to determine if cell senescence might be a contributing factor in the accelerated aging phenotype observed in the Sod1-/- mice. We focused on kidney because it is a tissue that has been shown to a significant increase in senescent cells with age. The Sod1-/- mice are characterized by high levels of DNA oxidation in the kidney, which is attenuated by DR. The kidney of the Sod1-/- mice also have higher levels of double strand DNA breaks than wild type (WT) mice. Expression (mRNA and protein) of p16 and p21, two of the markers of cellular senescence, which increased with age, are increased significantly in the kidney of Sod1-/- mice as is β-gal staining cells. In addition, the senescence associated secretory phenotype was also increased significantly in the kidney of Sod1-/- mice compared to WT mice as measured by the expression of transcripts for IL-6 and IL-1β. Dietary restriction of the Sod1-/- mice attenuated the increase in DNA damage, cellular senescence, and expression of IL-6 and IL-1β. Interestingly, the Sod1-/- mice showed higher levels of circulating cytokines than WT mice, suggesting that the accelerated aging phenotype shown by the Sod1-/- mice could result from increased inflammation arising from an accelerated accumulation of senescent cells. Based on our data with Sod1-/- mice, we propose that various bouts of increased oxidative stress over the lifespan of an animal leads to the accumulation of senescent cells. The accumulation of senescent cells in turn leads to increased inflammation, which plays a major role in the loss of function and increased pathology that are hallmark features of aging.
Keywords: Aging; Cellular senescence; DNA damage; Dietary restriction; Inflammation; Oxidative stress; Superoxide dismutase.
Copyright © 2016 The Authors. Published by Elsevier B.V. All rights reserved.
Figures


References
-
- Bokov A., Chaudhuri A., Richardson A. The role of oxidative damage and stress in aging. Mech. Ageing Dev. 2004;125:811–826. - PubMed
-
- Liang H., Masoro E.J., Nelson J.F., Strong R., McMahan C.A., Richardson A. Genetic mouse models of extended lifespan. Exp. Gerontol. 2003;38:1353–1364. - PubMed
-
- Elchuri S., Oberley T.D., Qi W., Eisenstein R.S., Jackson Roberts L., Van Remmen H., Epstein C.J., Huang T.T. CuZnSOD deficiency leads to persistent and widespread oxidative damage and hepatocarcinogenesis later in life. Oncogene. 2005;24:367–380. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

