Effect of concomitant use of memantine on mortality and efficacy outcomes of galantamine-treated patients with Alzheimer's disease: post-hoc analysis of a randomized placebo-controlled study
- PMID: 27846868
- PMCID: PMC5111338
- DOI: 10.1186/s13195-016-0214-x
Effect of concomitant use of memantine on mortality and efficacy outcomes of galantamine-treated patients with Alzheimer's disease: post-hoc analysis of a randomized placebo-controlled study
Abstract
Background: A large, prospective, 2-year, randomized study in patients with mild-to-moderate Alzheimer's disease or mixed dementia demonstrated reductions in mortality and cognitive/functional decline in galantamine-treated patients. A post-hoc analysis was conducted to study the effect of (the presence or absence of) concomitant memantine use on treatment outcome.
Methods: Randomized patients (N = 2045) were divided into subgroups based on memantine use. Analyses included demographic and clinical characteristics (age, nursing home placement, Mini-Mental State Examination (MMSE) and Disability Assessment for Dementia (DAD) scores) and mortality endpoints.
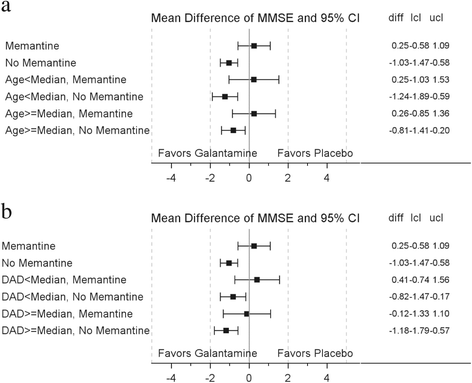
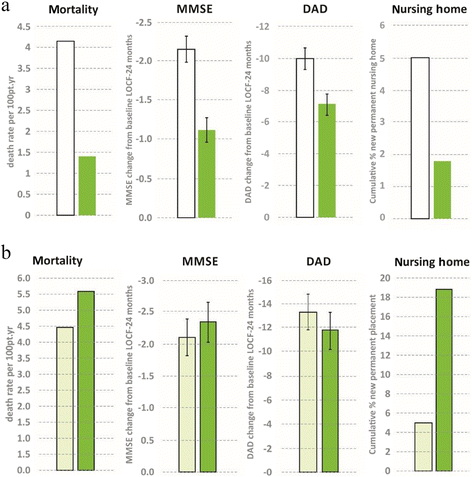
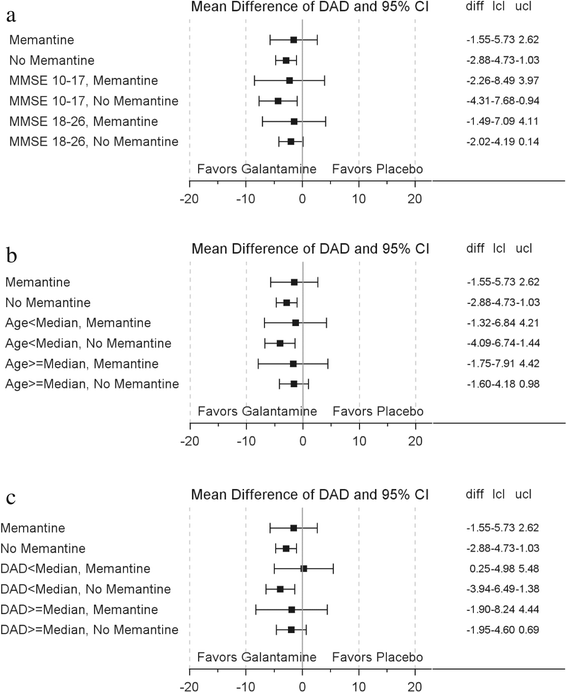
Results: Overall, 496 (24.3 %) patients were memantine users and were older (mean (SD), 74.0 (8.76) vs 72.8 (8.76), p = 0.008), with lower MMSE scores (18.2 (4.16) vs 19.2 (4.02), p < 0.0001) and DAD scores (58.0 (23.49) vs 62.5 (20.52), p < 0.0001) than nonusers. Mortality rates (per 100 patient-years) in memantine nonusers (n = 1549) were lower for galantamine (1.39) vs placebo-treated patients (4.15). In memantine users, mortality rates were similar for placebo-treated (4.49) and galantamine-treated patients (5.57). In memantine nonusers at 24 months, the decline in MMSE scores (effect size (95 % CI) 0.25 (0.14; 0.36)) and DAD scores (0.17 (0.06; 0.28)) from baseline was lower in galantamine patients vs placebo patients. The absence of these benefits in memantine users could not be explained by baseline age, MMSE, or DAD scores.
Conclusion: This post-hoc analysis shows that the beneficial effects of galantamine at 2 years post treatment were not observed in patients who had been placed on background memantine. The reasons for memantine treatment and the possibility of interaction between memantine and galantamine merit further investigation.
Trial registration: ClinicalTrials.gov NCT00679627 . Registered 15 May 2008.
Keywords: Alzheimer’s disease; Baseline scores; Galantamine; Memantine; Mortality.
Figures

References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

