Human Asymptomatic Epitopes Identified from the Herpes Simplex Virus Tegument Protein VP13/14 (UL47) Preferentially Recall Polyfunctional Effector Memory CD44high CD62Llow CD8+ TEM Cells and Protect Humanized HLA-A*02:01 Transgenic Mice against Ocular Herpesvirus Infection
- PMID: 27847359
- PMCID: PMC5215341
- DOI: 10.1128/JVI.01793-16
Human Asymptomatic Epitopes Identified from the Herpes Simplex Virus Tegument Protein VP13/14 (UL47) Preferentially Recall Polyfunctional Effector Memory CD44high CD62Llow CD8+ TEM Cells and Protect Humanized HLA-A*02:01 Transgenic Mice against Ocular Herpesvirus Infection
Abstract
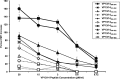
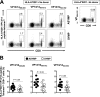
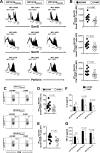
Herpes simplex virus 1 (HSV-1) infection is widespread among humans. The HSV-1 virion protein 13/14 (VP13/14), also known as UL47, is a tegument antigen targeted by CD8+ T cells from HSV-seropositive individuals. However, whether VP13/14-specific CD8+ T cells play a role in the natural protection seen in asymptomatic (ASYMP) individuals (individuals who have never had a clinical herpetic disease) has not been elucidated. Using predictive computer-assisted algorithms, we identified 10 potential HLA-A*02:01-restricted CD8+ T-cell epitopes from the 693-amino-acid sequence of the VP13/14 protein. Three out of 10 epitopes exhibited a high to moderate affinity of binding to soluble HLA-A*02:01 molecules. The phenotype and function of CD8+ T cells specific for each epitope were compared in HLA-A*02:01-positive ASYMP individuals and symptomatic (SYMP) individuals (individuals who have frequent clinical herpetic diseases) using determination of a combination of tetramer frequency and the levels of granzyme B, granzyme K, perforin, gamma interferon, tumor necrosis factor alpha, and interleukin-2 production and CD107a/b cytotoxic degranulation. High frequencies of multifunctional CD8+ T cells directed against three epitopes, VP13/14 from amino acids 286 to 294 (VP13/14286-294), VP13/14 from amino acids 504 to 512 (VP13/14504-512), and VP13/14 from amino acids 544 to 552 (VP13/14544-552), were detected in ASYMP individuals, while only low frequencies were detected in SYMP individuals. The three epitopes also predominantly recalled more CD45RAlow CD44high CCR7low CD62Llow CD8+ effector memory T cells (TEM cells) in ASYMP individuals than SYMP individuals. Moreover, immunization of HLA-A*02:01 transgenic mice with the three CD8+ TEM-cell epitopes from ASYMP individuals induced robust and polyfunctional HSV-specific CD8+ TEM cells associated with strong protective immunity against ocular herpesvirus infection and disease. Our findings outline the phenotypic and functional features of protective HSV-specific CD8+ T cells that should guide the development of a safe and effective T-cell-based herpes simplex vaccine.
Importance: Although most herpes simplex virus 1 (HSV-1)-infected individuals shed the virus in their body fluids following reactivation from latently infected sensory ganglia, the majority never develop a recurrent herpetic disease and remain asymptomatic (ASYMP). In contrast, small proportions of individuals are symptomatic (SYMP) and develop frequent bouts of recurrent disease. The present study demonstrates that naturally protected ASYMP individuals have a higher frequency of effector memory CD8+ T cells (CD8+ TEM cells) specific to three epitopes derived from the HSV-1 tegument protein VP13/14 (VP13/14286-294,VP13/14504-512, and VP13/14544-552) than SYMP patients. Moreover, immunization of humanized HLA-A*02:01 transgenic mice with the three CD8+ TEM-cell epitopes from ASYMP individuals induced robust and polyfunctional HSV-specific CD8+ T cells associated with strong protective immunity against ocular herpesvirus infection and disease. The findings support the emerging concept of the development of a safe and effective asymptomatic herpes simplex vaccine that is selectively based on CD8+ T-cell epitopes from ASYMP individuals.
Keywords: HSV; granzyme B; granzyme K; herpes simplex virus; human leukocyte antigen; humans; immunization; memory CD8+ T cells; perforin; transgenic; virion phosphoprotein.
Copyright © 2017 American Society for Microbiology.
Figures





References
-
- Srivastava R, Khan AA, Spencer D, Vahed H, Lopes PP, Thai NT, Wang C, Pham TT, Huang J, Scarfone VM, Nesburn AB, Wechsler SL, BenMohamed L. 2015. HLA-A02:01-restricted epitopes identified from the herpes simplex virus tegument protein VP11/12 preferentially recall polyfunctional effector memory CD8+ T cells from seropositive asymptomatic individuals and protect humanized HLA-A*02:01 transgenic mice against ocular herpes. J Immunol 194:2232–2248. doi:10.4049/jimmunol.1402606. - DOI - PMC - PubMed
-
- Samandary S, Kridane-Miledi H, Sandoval JS, Choudhury Z, Langa-Vives F, Spencer D, Chentoufi AA, Lemonnier FA, BenMohamed L. 2014. Associations of HLA-A, HLA-B and HLA-C alleles frequency with prevalence of herpes simplex virus infections and diseases across global populations: implication for the development of an universal CD8+ T-cell epitope-based vaccine. Hum Immunol 75:715–729. doi:10.1016/j.humimm.2014.04.016. - DOI - PMC - PubMed
-
- Dervillez X, Qureshi H, Chentoufi AA, Khan AA, Kritzer E, Yu DC, Diaz OR, Gottimukkala C, Kalantari M, Villacres MC, Scarfone VM, McKinney DM, Sidney J, Sette A, Nesburn AB, Wechsler SL, BenMohamed L. 2013. Asymptomatic HLA-A*02:01-restricted epitopes from herpes simplex virus glycoprotein B preferentially recall polyfunctional CD8+ T cells from seropositive asymptomatic individuals and protect HLA transgenic mice against ocular herpes. J Immunol 191:5124–5138. doi:10.4049/jimmunol.1301415. - DOI - PMC - PubMed
-
- BenMohamed L, Osorio N, Srivastava R, Khan AA, Simpson JL, Wechsler SL. 2015. Decreased reactivation of a herpes simplex virus type 1 (HSV-1) latency-associated transcript (LAT) mutant using the in vivo mouse UV-B model of induced reactivation. J Neurovirol 21:508–517. doi:10.1007/s13365-015-0348-9. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

