The Live Attenuated Cholera Vaccine CVD 103-HgR Primes Responses to the Toxin-Coregulated Pilus Antigen TcpA in Subjects Challenged with Wild-Type Vibrio cholerae
- PMID: 27847368
- PMCID: PMC5216439
- DOI: 10.1128/CVI.00470-16
The Live Attenuated Cholera Vaccine CVD 103-HgR Primes Responses to the Toxin-Coregulated Pilus Antigen TcpA in Subjects Challenged with Wild-Type Vibrio cholerae
Abstract
One potential advantage of live attenuated bacterial vaccines is the ability to stimulate responses to antigens which are only expressed during the course of infection. To determine whether the live attenuated cholera vaccine CVD 103-HgR (Vaxchora) results in antibody responses to the in vivo-induced toxin-coregulated pilus antigen TcpA, we measured IgA and IgG responses to Vibrio cholerae O1 El Tor TcpA in a subset of participants in a recently reported experimental challenge study. Participants were challenged with V. cholerae O1 El Tor Inaba N16961 either 10 days or 90 days after receiving the vaccine or a placebo. Neither vaccination nor experimental infection with V. cholerae alone resulted in a robust TcpA IgG or IgA response, but each did elicit a strong response to cholera toxin. However, compared to placebo recipients, vaccinees had a marked increase in IgG TcpA antibodies following the 90-day challenge, suggesting that vaccination with CVD 103-HgR resulted in priming for a subsequent response to TcpA. No such difference between vaccine and placebo recipients was observed for volunteers challenged 10 days after vaccination, indicating that this was insufficient time for vaccine-induced priming of the TcpA response. The priming of the response to TcpA and potentially other antigens expressed in vivo by attenuated V. cholerae may have relevance to the maintenance of immunity in areas where cholera is endemic.
Keywords: TcpA; challenge; cholera; priming; vaccines.
Copyright © 2017 American Society for Microbiology.
Figures
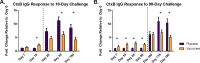



Similar articles
-
Anti-O-specific polysaccharide (OSP) immune responses following vaccination with oral cholera vaccine CVD 103-HgR correlate with protection against cholera after infection with wild-type Vibrio cholerae O1 El Tor Inaba in North American volunteers.PLoS Negl Trop Dis. 2018 Apr 6;12(4):e0006376. doi: 10.1371/journal.pntd.0006376. eCollection 2018 Apr. PLoS Negl Trop Dis. 2018. PMID: 29624592 Free PMC article. Clinical Trial.
-
Transcutaneous immunization with toxin-coregulated pilin A induces protective immunity against Vibrio cholerae O1 El Tor challenge in mice.Infect Immun. 2006 Oct;74(10):5834-9. doi: 10.1128/IAI.00438-06. Infect Immun. 2006. PMID: 16988262 Free PMC article.
-
Intranasal immunization with recombinant toxin-coregulated pilus and cholera toxin B subunit protects rabbits against Vibrio cholerae O1 challenge.FEMS Immunol Med Microbiol. 2009 Jul;56(2):179-84. doi: 10.1111/j.1574-695X.2009.00563.x. Epub 2009 May 5. FEMS Immunol Med Microbiol. 2009. PMID: 19453752
-
PaxVax CVD 103-HgR single-dose live oral cholera vaccine.Expert Rev Vaccines. 2017 Mar;16(3):197-213. doi: 10.1080/14760584.2017.1291348. Expert Rev Vaccines. 2017. PMID: 28165831 Review.
-
A review of the current status of enteric vaccines.P N G Med J. 1995 Dec;38(4):325-31. P N G Med J. 1995. PMID: 9522876 Review.
Cited by
-
Identifying Recent Cholera Infections Using a Multiplex Bead Serological Assay.mBio. 2022 Dec 20;13(6):e0190022. doi: 10.1128/mbio.01900-22. Epub 2022 Oct 26. mBio. 2022. PMID: 36286520 Free PMC article.
-
Vibrio cholerae at the Intersection of Immunity and the Microbiome.mSphere. 2019 Nov 27;4(6):e00597-19. doi: 10.1128/mSphere.00597-19. mSphere. 2019. PMID: 31776240 Free PMC article. Review.
-
Controlled Human Infection Models To Accelerate Vaccine Development.Clin Microbiol Rev. 2022 Sep 21;35(3):e0000821. doi: 10.1128/cmr.00008-21. Epub 2022 Jul 6. Clin Microbiol Rev. 2022. PMID: 35862754 Free PMC article. Review.
-
Vibrio cholerae, classification, pathogenesis, immune response, and trends in vaccine development.Front Med (Lausanne). 2023 May 5;10:1155751. doi: 10.3389/fmed.2023.1155751. eCollection 2023. Front Med (Lausanne). 2023. PMID: 37215733 Free PMC article. Review.
-
From ancient remedies to modern miracles: tracing the evolution of vaccines and their impact on public health.3 Biotech. 2024 Oct;14(10):242. doi: 10.1007/s13205-024-04075-7. Epub 2024 Sep 22. 3 Biotech. 2024. PMID: 39319014 Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

