Emergence of Multidrug-Resistant Pneumococcal Serotype 35B among Children in the United States
- PMID: 27847379
- PMCID: PMC5328440
- DOI: 10.1128/JCM.01778-16
Emergence of Multidrug-Resistant Pneumococcal Serotype 35B among Children in the United States
Abstract
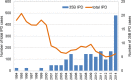
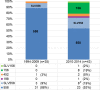
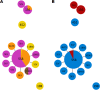
Streptococcus pneumoniae serotype 35B is a nonvaccine serotype associated with high rates of penicillin nonsusceptibility. An increase in the proportion of multidrug-resistant (MDR) 35B isolates has recently been reported. The genetic events contributing to the emergence of MDR serotype 35B are unknown. The sequence type (ST) composition of 78 serotype 35B isolates obtained from pediatric patients with invasive pneumococcal disease from 1994 to 2014 and 48 isolates from pediatric patients with otitis media (noninvasive) from 2011 to 2014 was characterized by multilocus sequence typing (MLST). The most common STs were ST558 (69.2%), ST156 (10.3%), and ST452 (3.8%). Two major clonal complexes (CC), CC558 and CC156, were identified by eBURST analysis. Overall, 91% (71/78) of isolates were penicillin nonsusceptible and 16.7% (13/78) were MDR. Among all invasive serotype 35B isolates, MDR isolates increased significantly, from 2.9% (1/35) to 27.9% (12/43) (P = 0.004), after the 13-valent pneumococcal conjugate vaccine (PCV13) was introduced. All CC156 isolates were identified after the introduction of PCV13 (0/35 [0%] before versus 9/43 [20.9%] after; P = 0.003) and were MDR. All CC156 isolates had similar antimicrobial susceptibility patterns; in contrast, high variability in antimicrobial susceptibility was observed among CC558 isolates. The distributions of CC558 and CC156 among invasive and noninvasive isolates were not different. The increased prevalence of MDR serotype 35B after the introduction of PCV13 was directly associated with the emergence of ST156. Genotyping suggests that capsular switching has occurred between MDR vaccine serotypes belonging to ST156 (e.g., 9V, 14, and 19A) and serotype 35B.
Keywords: Streptococcus pneumoniae; multidrug resistance; otitis media; pneumococcal disease; pneumococcal vaccine; pneumococcus; serotype 35B.
Copyright © 2017 American Society for Microbiology.
Figures

Comment in
-
The Ongoing Genetic Adaptation of Streptococcus pneumoniae.J Clin Microbiol. 2017 Mar;55(3):681-685. doi: 10.1128/JCM.02283-16. Epub 2016 Dec 14. J Clin Microbiol. 2017. PMID: 27974545 Free PMC article.
References
-
- Kaplan SL, Barson WJ, Lin PL, Romero JR, Bradley JS, Tan TQ, Hoffman JA, Givner LB, Mason EO. 2013. Early trends for invasive pneumococcal infections in children after the introduction of the 13-valent pneumococcal conjugate vaccine. Pediatr Infect Dis J 32:203–207. doi:10.1097/INF.0b013e318275614b. - DOI - PubMed
-
- Moore MR, Link-Gelles R, Schaffner W, Lynfield R, Lexau C, Bennett NM, Petit S, Zansky SM, Harrison LH, Reingold A, Miller L, Scherzinger K, Thomas A, Farley MM, Zell ER, Taylor TH, Pondo T, Rodgers L, McGee L, Beall B, Jorgensen JH, Whitney CG. 2015. Effect of use of 13-valent pneumococcal conjugate vaccine in children on invasive pneumococcal disease in children and adults in the USA: analysis of multisite, population-based surveillance. Lancet Infect Dis 15:301–309. doi:10.1016/S1473-3099(14)71081-3. - DOI - PMC - PubMed
-
- Kaplan SL, Center KJ, Barson WJ, Ling-Lin P, Romero JR, Bradley JS, Tan TQ, Hoffman JA, Peters TR, Gurtman A, Scott DA, Trammel J, Gruber WC, Hulten KG, Mason EO. 2015. Multicenter surveillance of Streptococcus pneumoniae isolates from middle ear and mastoid cultures in the 13-valent pneumococcal conjugate vaccine era. Clin Infect Dis 60:1339–1345. doi:10.1093/cid/civ067. - DOI - PubMed
-
- Desai AP, Sharma D, Crispell EK, Baughman W, Thomas S, Tunali A, Sherwood L, Zmitrovich A, Jerris R, Satola S, Beall B, Moore MR, Jain S, Farley MM. 2015. Decline in pneumococcal nasopharyngeal carriage of vaccine serotypes after the introduction of the 13-valent pneumococcal conjugate vaccine in children in Atlanta, Georgia. Pediatr Infect Dis J 34:1168–1174. doi:10.1097/INF.0000000000000849. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

