High Mobility Group Box-1: A Missing Link between Diabetes and Its Complications
- PMID: 27847406
- PMCID: PMC5099456
- DOI: 10.1155/2016/3896147
High Mobility Group Box-1: A Missing Link between Diabetes and Its Complications
Abstract
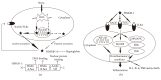
High mobility group box-1 (HMGB-1), a damage-associated molecular pattern, can be actively or passively released from various cells under different conditions and plays a pivotal role in the pathogenesis of inflammation and angiogenesis-dependent diseases. More and more evidence suggests that inflammation, in addition to its role in progression of diabetes, also promotes initiation and development of diabetic complications. In this review, we focus on the role of HMGB-1 in diabetes-related complications and the therapeutic strategies targeting HMGB-1 in diabetic complications.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

