Chicken IgY Fc Linked to Bordetella avium ompA and Taishan Pinus massoniana Pollen Polysaccharide Adjuvant Enhances Macrophage Function and Specific Immune Responses
- PMID: 27847501
- PMCID: PMC5088198
- DOI: 10.3389/fmicb.2016.01708
Chicken IgY Fc Linked to Bordetella avium ompA and Taishan Pinus massoniana Pollen Polysaccharide Adjuvant Enhances Macrophage Function and Specific Immune Responses
Abstract
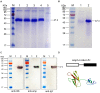
Fc-fusion technologies, in which immunoglobulin Fc is genetically fused to an antigenic protein, have been developed to confer antibody-like properties to proteins and peptides. Mammalian IgG Fc fusion exhibits improved antigen-induced immune responses by providing aggregates with high avidity for the IgG Fc receptor and salvaging the antigenic portion from endosomal degradation. However, whether the linked chicken IgY Fc fragment shares similar characteristics to mammalian IgG Fc remains unclear. In this study, we linked the chicken IgY Fc gene to the outer membrane protein A (ompA) of Bordetella avium through overlapping PCR. The fusion gene was cloned into the pPIC9 plasmid to construct the recombinant Pichia pastoris transformant expressing the ompA-Fc fusion protein. The effects of the linked Fc on macrophage vitality, activity, efficiency of antigen processing, and immune responses induced by the fused ompA were investigated. Furthermore, the effect of Taishan Pinus massoniana pollen polysaccharide (TPPPS), an immunomodulator, on chicken macrophage activation was evaluated. TPPPS was also used as an adjuvant to investigate its immunomodulatory effect on immunoresponses induced by the fused ompA-Fc in chickens. The pinocytosis, phagocytosis, secretion of nitric oxide and TNF-α, and MHC-II molecular expression of the macrophages treated with the fused ompA-Fc were significantly higher than those of the macrophages treated with ompA alone. The addition of TPPPS to the fused ompA-Fc further enhanced macrophage functions. The fused ompA-Fc elicited higher antigen-specific immune responses and protective efficacy compared with ompA alone. Moreover, the fused ompA-Fc conferred higher serum antibody titers, serum IL-2 and IL-4 concentrations, CD4+ and CD8+ T-lymphocyte counts, lymphocyte transformation rate, and protection rate compared with ompA alone. Notably, the prepared TPPPS adjuvant ompA-Fc vaccines induced high immune responses and protection rate. The linked Fc and TPPPS adjuvant can remarkably enhance macrophage functions and specific immune responses. This study provides new perspectives to improve the immune effects of subunit vaccines for prevention of poultry diseases.
Keywords: IgY Fc; Pichia pastoris expression; TPPPS; peritoneal macrophage; subunit vaccine.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

