Fusion proteins for treatment of retinal diseases: aflibercept, ziv-aflibercept, and conbercept
- PMID: 27847621
- PMCID: PMC5088480
- DOI: 10.1186/s40942-016-0026-y
Fusion proteins for treatment of retinal diseases: aflibercept, ziv-aflibercept, and conbercept
Abstract
In the last few years, monoclonal antibodies have revolutionized the treatment of retinal neovascular diseases. More recently, a different class of drugs, fusion proteins, has provided an alternative treatment strategy with pharmacological differences. In addition to commercially available aflibercept, two other drugs, ziv-aflibercept and conbercept, have been studied in antiangiogenic treatment of ocular diseases. In this scenario, a critical review of the currently available data regarding fusion proteins in ophthalmic diseases may be a timely and important contribution. Aflibercept, previously known as VEGF Trap Eye, is a fusion protein of VEGF receptors 1 and 2 and a treatment for several retinal diseases related to angiogenesis. It has firmly joined ranibizumab and bevacizumab as an important therapeutic option in the management of neovascular AMD-, DME- and RVO-associated macular edema. Ziv-aflibercept, a systemic chemotherapeutic agent approved for the treatment of metastatic colorectal cancer, has recently drawn attention because of its potential for intravitreal administration, since it was not associated with ERG-related signs of toxicity in an experimental study and in human case reports. Conbercept is a soluble receptor decoy that blocks all isoforms of VEGF-A, VEGF-B, VEGF-C, and PlGF, which has a high binding affinity for VEGF and a long half-life in vitreous. It has been studied in a phase three clinical trial and has shown efficacy and safety. This review discusses three fusion proteins that have been studied in ophthalmology, aflibercept, ziv-aflibercept and conbercept, with emphasis on their clinical application for the treatment of retinal diseases.
Keywords: Aflibercept; Conbercept; Fusion proteins; VEGF Trap Eye; Vascular endothelial growth factor (VEGF); Ziv-aflibercept.
Figures
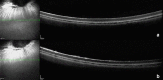
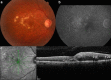
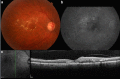
References
-
- Zachary I. Vascular endothelial growth factor and anti-angiogenic peptides as therapeutic and investigational molecules. IDrugs. 2003;6:224–231. - PubMed
-
- Glaser BM, D’Amore PA, Lutty GA, Fenselau AH, Michels RG, Patz A. Chemical mediators of intraocular neovascularization. Trans Ophthalmol Soc. 1980;100:369–373. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

