The role of optical coherence tomography in Alzheimer's disease
- PMID: 27847642
- PMCID: PMC5088456
- DOI: 10.1186/s40942-016-0049-4
The role of optical coherence tomography in Alzheimer's disease
Abstract
Background: Alzheimer's disease (AD) is the most common cause of dementia and its incidence is increasing worldwide along with population aging. Previous clinical and histologic studies suggest that the neurodegenerative process, which affects the brain, may also affect the retina of AD patients.
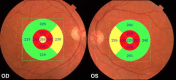
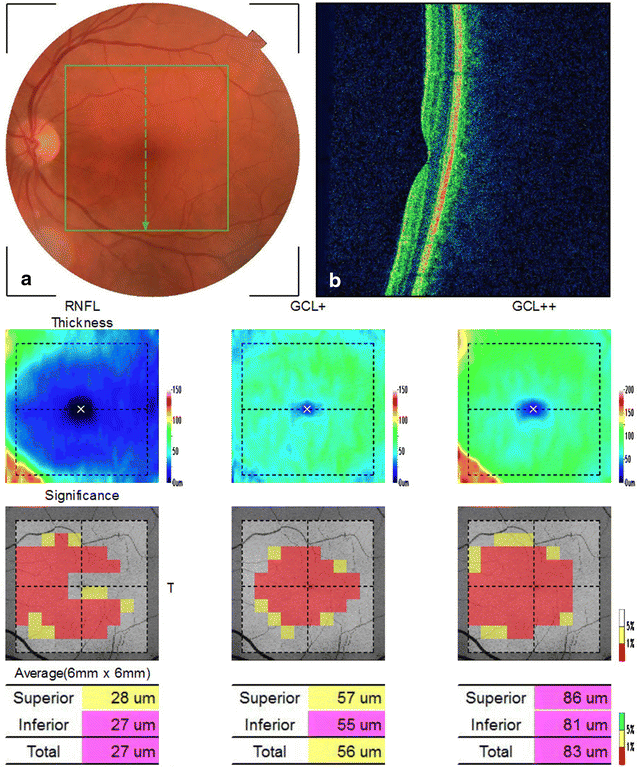
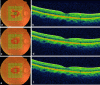
Main body: Optical coherence tomography (OCT) is a non-invasive technology that acquires cross-sectional images of retinal structures allowing neural fundus integrity assessment. Several previous studies demonstrated that both peripapillary retinal nerve fiber layer and macular thickness measurements assessed by OCT were able to detect neuronal loss in AD. Moreover, recent advances in OCT technology, have allowed substantial enhancement in ultrastructural evaluation of the macula, enabling the assessment not only of full-thickness retinal measurements but also of inner retinal layers, which seems to be a promising approach, mainly regarding the assessment of retinal ganglion cell layer impairment in AD patients. Furthermore, retinal neuronal loss seems to correlate with cognitive impairment in AD, reinforcing the promising role of OCT in the clinical evaluation of these patients.
Conclusion: The purpose of this article is to review the main findings on OCT in AD patients, to discuss the role of this important diagnostic tool in these patients and how OCT technology may be useful in understanding morphological retinal changes in AD.
Keywords: Alzheimer’s disease; Dementia; Ganglion cell layer; Macula; Mild cognitive impairment; Optic nerve; Optical coherence tomography; Retina; Retinal nerve fiber layer.
Figures
References
-
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/WNL.34.7.939. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

