Aqueous Angiography with Fluorescein and Indocyanine Green in Bovine Eyes
- PMID: 27847692
- PMCID: PMC5106193
- DOI: 10.1167/tvst.5.6.5
Aqueous Angiography with Fluorescein and Indocyanine Green in Bovine Eyes
Abstract
Purpose: We characterize aqueous angiography as a real-time aqueous humor outflow imaging (AHO) modality in cow eyes with two tracers of different molecular characteristics.
Methods: Cow enucleated eyes (n = 31) were obtained and perfused with balanced salt solution via a Lewicky AC maintainer through a 1-mm side-port. Fluorescein (2.5%) or indocyanine green (ICG; 0.4%) were introduced intracamerally at 10 mm Hg individually or sequentially. With an angiographer, infrared and fluorescent images were acquired. Concurrent anterior segment optical coherence tomography (OCT) was performed, and fixable fluorescent dextrans were introduced into the eye for histologic analysis of angiographically positive and negative areas.
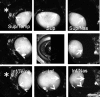
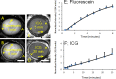
Results: Aqueous angiography in cow eyes with fluorescein and ICG yielded high-quality images with segmental patterns. Over time, ICG maintained a better intraluminal presence. Angiographically positive, but not negative, areas demonstrated intrascleral lumens with anterior segment OCT. Aqueous angiography with fluorescent dextrans led to their trapping in AHO pathways. Sequential aqueous angiography with ICG followed by fluorescein in cow eyes demonstrated similar patterns.
Conclusions: Aqueous angiography in model cow eyes demonstrated segmental angiographic outflow patterns with either fluorescein or ICG as a tracer.
Translational relevance: Further characterization of segmental AHO with aqueous angiography may allow for intelligent placement of trabecular bypass minimally invasive glaucoma surgeries for improved surgical results.
Keywords: angiography; aqueous angiography; fluorescein angiography; minimally invasive glaucoma surgeries.
Figures







References
-
- Craven ER,, Katz LJ,, Wells JM,, Giamporcaro JE. Cataract surgery with trabecular micro-bypass stent implantation in patients with mild-to-moderate open-angle glaucoma and cataract: two-year follow-up. J Cataract Refract Surg. 2012. ; 38: 1339–1345. - PubMed
-
- Chang JY,, Folz SJ,, Laryea SN,, Overby DR. Multi-scale analysis of segmental outflow patterns in human trabecular meshwork with changing intraocular pressure. J Ocul Pharmacol Ther. 2014. ; 30: 213–223. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

