Diet, microorganisms and their metabolites, and colon cancer
- PMID: 27848961
- PMCID: PMC6312102
- DOI: 10.1038/nrgastro.2016.165
Diet, microorganisms and their metabolites, and colon cancer
Abstract
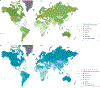
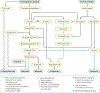
Colorectal cancer is one of the so-called westernized diseases and the second leading cause of cancer death worldwide. On the basis of global epidemiological and scientific studies, evidence suggests that the risk of colorectal cancer is increased by processed and unprocessed meat consumption but suppressed by fibre, and that food composition affects colonic health and cancer risk via its effects on colonic microbial metabolism. The gut microbiota can ferment complex dietary residues that are resistant to digestion by enteric enzymes. This process provides energy for the microbiota but culminates in the release of short-chain fatty acids including butyrate, which are utilized for the metabolic needs of the colon and the body. Butyrate has a remarkable array of colonic health-promoting and antineoplastic properties: it is the preferred energy source for colonocytes, it maintains mucosal integrity and it suppresses inflammation and carcinogenesis through effects on immunity, gene expression and epigenetic modulation. Protein residues and fat-stimulated bile acids are also metabolized by the microbiota to inflammatory and/or carcinogenic metabolites, which increase the risk of neoplastic progression. This Review will discuss the mechanisms behind these microbial metabolite effects, which could be modified by diet to achieve the objective of preventing colorectal cancer in Western societies.
Conflict of interest statement
Competing interests statement
The author declares no competing interests.
Figures

References
-
- Ferlay J et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [online]. Lyon, France: International Agency for Research on Cancer, http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx?cancer=colorectal (2013).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

