Cell senescence is an antiviral defense mechanism
- PMID: 27849057
- PMCID: PMC5111111
- DOI: 10.1038/srep37007
Cell senescence is an antiviral defense mechanism
Abstract
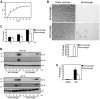
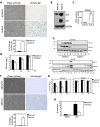
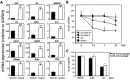
Cellular senescence is often considered a protection mechanism triggered by conditions that impose cellular stress. Continuous proliferation, DNA damaging agents or activated oncogenes are well-known activators of cell senescence. Apart from a characteristic stable cell cycle arrest, this response also involves a proinflammatory phenotype known as senescence-associated secretory phenotype (SASP). This, together with the widely known interference with senescence pathways by some oncoviruses, had led to the hypothesis that senescence may also be part of the host cell response to fight virus. Here, we evaluate this hypothesis using vesicular stomatitis virus (VSV) as a model. Our results show that VSV replication is significantly impaired in both primary and tumor senescent cells in comparison with non-senescent cells, and independently of the stimulus used to trigger senescence. Importantly, we also demonstrate a protective effect of senescence against VSV in vivo. Finally, our results identify the SASP as the major contributor to the antiviral defense exerted by cell senescence in vitro, and points to a role activating and recruiting the immune system to clear out the infection. Thus, our study indicates that cell senescence has also a role as a natural antiviral defense mechanism.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
-
- Hayflick L. & Moorhead P. S. The serial cultivation of human diploid cell strains. Exp Cell Res 25, 585–621 (1961). - PubMed
-
- Collado M., Blasco M. A. & Serrano M. Cellular senescence in cancer and aging. Cell 130, 223–233 (2007). - PubMed
-
- Munoz-Espin D. et al. Programmed cell senescence during mammalian embryonic development. Cell 155, 1104–1118 (2013). - PubMed
-
- Storer M. et al. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell 155, 1119–1130 (2013). - PubMed
-
- Munoz-Espin D. & Serrano M. Cellular senescence: from physiology to pathology. Nat Rev Mol Cell Biol 15, 482–496 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

