Modulation of Pertussis and Adenylate Cyclase Toxins by Sigma Factor RpoE in Bordetella pertussis
- PMID: 27849178
- PMCID: PMC5203664
- DOI: 10.1128/IAI.00565-16
Modulation of Pertussis and Adenylate Cyclase Toxins by Sigma Factor RpoE in Bordetella pertussis
Abstract
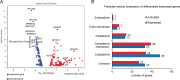
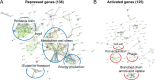
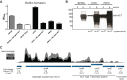
Bordetella pertussis is a human pathogen that can infect the respiratory tract and cause the disease known as whooping cough. B. pertussis uses pertussis toxin (PT) and adenylate cyclase toxin (ACT) to kill and modulate host cells to allow the pathogen to survive and persist. B. pertussis encodes many uncharacterized transcription factors, and very little is known about their functions. RpoE is a sigma factor which, in other bacteria, responds to oxidative, heat, and other environmental stresses. RseA is a negative regulator of RpoE that sequesters the sigma factor to regulate gene expression based on conditions. In B. pertussis, deletion of the rseA gene results in high transcriptional activity of RpoE and large amounts of secretion of ACT. By comparing parental B. pertussis to an rseA gene deletion mutant (PM18), we sought to characterize the roles of RpoE in virulence and determine the regulon of genes controlled by RpoE. Despite high expression of ACT, the rseA mutant strain did not infect the murine airway as efficiently as the parental strain and PM18 was killed more readily when inside phagocytes. RNA sequencing analysis was performed and 263 genes were differentially regulated by RpoE, and surprisingly, the rseA mutant strain where RpoE activity was elevated expressed very little pertussis toxin. Western blots and proteomic analysis corroborated the inverse relationship of PT to ACT expression in the high-RpoE-activity rseA deletion strain. Our data suggest that RpoE can modulate PT and ACT expression indirectly through unidentified mechanisms in response to conditions.
Keywords: Bordetella pertussis; RpoE; adenylate cyclase toxin; pertussis toxin.
Copyright © 2016 American Society for Microbiology.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

