Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma
- PMID: 27849562
- PMCID: PMC5512174
- DOI: 10.1136/gutjnl-2016-312268
Palbociclib (PD-0332991), a selective CDK4/6 inhibitor, restricts tumour growth in preclinical models of hepatocellular carcinoma
Abstract
Objective: Advanced hepatocellular carcinoma (HCC) is a lethal malignancy with limited treatment options. Palbociclib, a well-tolerated and selective CDK4/6 inhibitor, has shown promising results in the treatment of retinoblastoma (RB1)-positive breast cancer. RB1 is rarely mutated in HCC, suggesting that palbociclib could potentially be used for HCC therapy. Here, we provide a comprehensive characterisation of the efficacy of palbociclib in multiple preclinical models of HCC.
Design: The effects of palbociclib on cell proliferation, cellular senescence and cell death were investigated in a panel of human liver cancer cell lines, in ex vivo human HCC samples, in a genetically engineered mouse model of liver cancer, and in human HCC xenografts in vivo. The mechanisms of intrinsic and acquired resistance to palbociclib were assessed in human liver cancer cell lines and human HCC samples by protein and gene expression analyses.
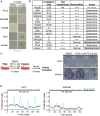
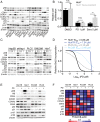
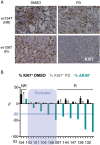
Results: Palbociclib suppressed cell proliferation in human liver cancer cell lines by promoting a reversible cell cycle arrest. Intrinsic and acquired resistance to palbociclib was determined by loss of RB1. A signature of 'RB1 loss of function' was found in <30% of HCC samples. Palbociclib, alone or combined with sorafenib, the standard of care for HCC, impaired tumour growth in vivo and significantly increased survival.
Conclusions: Palbociclib shows encouraging results in preclinical models of HCC and represents a novel therapeutic strategy for HCC treatment, alone or particularly in combination with sorafenib. Palbociclib could potentially benefit patients with RB1-proficient tumours, which account for 70% of all patients with HCC.
Keywords: CELL CYCLE; HEPATOCELLULAR CARCINOMA.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
Competing interests: None declared.
Figures





Comment in
-
CDK4/6 inhibition and sorafenib: a ménage à deux in HCC therapy?Gut. 2017 Jul;66(7):1179-1180. doi: 10.1136/gutjnl-2016-313547. Epub 2017 Jan 5. Gut. 2017. PMID: 28057692 No abstract available.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous
