Quorum sensing controls the Pseudomonas aeruginosa CRISPR-Cas adaptive immune system
- PMID: 27849583
- PMCID: PMC5224376
- DOI: 10.1073/pnas.1617415113
Quorum sensing controls the Pseudomonas aeruginosa CRISPR-Cas adaptive immune system
Abstract
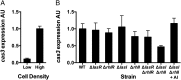
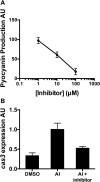
CRISPR-Cas are prokaryotic adaptive immune systems that provide protection against bacteriophage (phage) and other parasites. Little is known about how CRISPR-Cas systems are regulated, preventing prediction of phage dynamics in nature and manipulation of phage resistance in clinical settings. Here, we show that the bacterium Pseudomonas aeruginosa PA14 uses the cell-cell communication process, called quorum sensing, to activate cas gene expression, to increase CRISPR-Cas targeting of foreign DNA, and to promote CRISPR adaptation, all at high cell density. This regulatory mechanism ensures maximum CRISPR-Cas function when bacterial populations are at highest risk for phage infection. We demonstrate that CRISPR-Cas activity and acquisition of resistance can be modulated by administration of pro- and antiquorum-sensing compounds. We propose that quorum-sensing inhibitors could be used to suppress the CRISPR-Cas adaptive immune system to enhance medical applications, including phage therapies.
Keywords: CRISPR; immunity; phage; phage defense; quorum sensing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Comment in
-
Bacterial Physiology: Quorum sensing controls the cost of CRISPR-Cas.Nat Rev Microbiol. 2017 Jan;15(1):2-3. doi: 10.1038/nrmicro.2016.180. Epub 2016 Nov 28. Nat Rev Microbiol. 2017. PMID: 27890921 No abstract available.
-
Sensing danger.Proc Natl Acad Sci U S A. 2017 Jan 3;114(1):15-16. doi: 10.1073/pnas.1618747114. Epub 2016 Dec 20. Proc Natl Acad Sci U S A. 2017. PMID: 27999179 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

