Gram-positive bacteria are held at a distance in the colon mucus by the lectin-like protein ZG16
- PMID: 27849619
- PMCID: PMC5137749
- DOI: 10.1073/pnas.1611400113
Gram-positive bacteria are held at a distance in the colon mucus by the lectin-like protein ZG16
Abstract
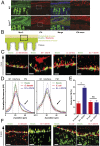
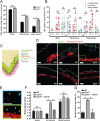
The distal colon functions as a bioreactor and harbors an enormous amount of bacteria in a mutualistic relationship with the host. The microbiota have to be kept at a safe distance to prevent inflammation, something that is achieved by a dense inner mucus layer that lines the epithelial cells. The large polymeric nets made up by the heavily O-glycosylated MUC2 mucin forms this physical barrier. Proteomic analyses of mucus have identified the lectin-like protein ZG16 (zymogen granulae protein 16) as an abundant mucus component. To elucidate the function of ZG16, we generated recombinant ZG16 and studied Zg16-/- mice. ZG16 bound to and aggregated Gram-positive bacteria via binding to the bacterial cell wall peptidoglycan. Zg16-/- mice have a distal colon mucus layer with normal thickness, but with bacteria closer to the epithelium. Using distal colon explants mounted in a horizontal perfusion chamber we demonstrated that treatment of bacteria with recombinant ZG16 hindered bacterial penetration into the mucus. The inner colon mucus of Zg16-/- animals had a higher load of Gram-positive bacteria and showed bacteria with higher motility in the mucus close to the host epithelium compared with cohoused littermate Zg16+/+ The more penetrable Zg16-/- mucus allowed Gram-positive bacteria to translocate to systemic tissues. Viable bacteria were found in spleen and were associated with increased abdominal fat pad mass in Zg16-/- animals. The function of ZG16 reveals a mechanism for keeping bacteria further away from the host colon epithelium.
Keywords: colon; inflammation; mucin; obesity; peptidoglycan.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307(5717):1915–1920. - PubMed
-
- Ermund A, Schütte A, Johansson MEV, Gustafsson JK, Hansson GC. Studies of mucus in mouse stomach, small intestine, and colon. I. Gastrointestinal mucus layers have different properties depending on location as well as over the Peyer’s patches. Am J Physiol Gastrointest Liver Physiol. 2013;305(5):G341–G347. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

