Atorvastatin Alleviates Experimental Diabetic Cardiomyopathy by Regulating the GSK-3β-PP2Ac-NF-κB Signaling Axis
- PMID: 27851811
- PMCID: PMC5112957
- DOI: 10.1371/journal.pone.0166740
Atorvastatin Alleviates Experimental Diabetic Cardiomyopathy by Regulating the GSK-3β-PP2Ac-NF-κB Signaling Axis
Abstract
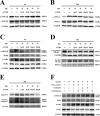
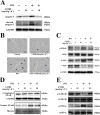
Recent studies reported that atorvastatin (ATOR) alleviated progression of experimental diabetic cardiomyopathy (DCM), possibly by protecting against apoptosis. However, the underlying mechanisms of this protective effect remain unclear. Therefore, our study investigated the role of the glycogen synthase kinase (GSK)-3β-protein phosphatase 2A(PP2A)-NF-κB signaling pathway in the anti-apoptotic and cardioprotective effects of ATOR on cardiomyocytes cultured in high glucose (HG) and in DCM. Our results showed that, in HG-cultured cardiomyocytes, phosphorylation of GSK-3β was decreased, while that of the PP2A catalytic subunit C (PP2Ac) and IKK/IкBα was increased, followed by NF-кB nuclear translocation and apoptosis. IKK/IкBα phosphorylation and NF-кB nuclear translocation were also increased by treatment of cells with okadaic acid (OA), a selective PP2A inhibitor, or by silencing PP2Ac expression. The opposite results were obtained by silencing GSK-3β expression, which resulted in PP2Ac activation. Furthermore, IKK/IкBα phosphorylation and NF-кB nuclear translocation were markedly inhibited and apoptosis attenuated in cells treated with ATOR. These effects occurred through inactivation of GSK-3β and subsequent activation of PP2Ac. They were abolished by treatment of cells with OA or PP2Ac siRNA. In mice with type 1 diabetes mellitus, treatment with ATOR, at 10 mg-kg-1-d-1, significantly suppressed GSK-3β activation, IKK/IкBα phosphorylation, NF-кB nuclear translocation and caspase-3 activation, while also activating PP2Ac. Finally, improvements in histological abnormalities, fibrosis, apoptosis and cardiac dysfunction were observed in diabetic mice treated with ATOR. These findings demonstrated that ATOR protected against HG-induced apoptosis in cardiomyocytes and alleviated experimental DCM by regulating the GSK-3β-PP2A-NF-κB signaling pathway.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Activation of the PP2A catalytic subunit by ivabradine attenuates the development of diabetic cardiomyopathy.J Mol Cell Cardiol. 2019 May;130:170-183. doi: 10.1016/j.yjmcc.2019.04.011. Epub 2019 Apr 15. J Mol Cell Cardiol. 2019. PMID: 30998977
-
High-glucose-induced apoptosis, ROS production and pro-inflammatory response in cardiomyocytes is attenuated by metformin treatment via PP2A activation.J Biosci. 2020;45:126. J Biosci. 2020. PMID: 33184242
-
Retinoic acid protects cardiomyocytes from high glucose-induced apoptosis through inhibition of NF-κB signaling pathway.J Cell Physiol. 2013 Feb;228(2):380-92. doi: 10.1002/jcp.24142. J Cell Physiol. 2013. PMID: 22718360 Free PMC article.
-
[Participation of heat shock proteins in modulation of NF-кB transcription factor activation during bacterial infections].Postepy Hig Med Dosw (Online). 2015 Jan 2;69:969-77. doi: 10.5604/17322693.1165199. Postepy Hig Med Dosw (Online). 2015. PMID: 26400883 Review. Polish.
-
Diabetic cardiomyopathy - Zinc preventive and therapeutic potentials by its anti-oxidative stress and sensitizing insulin signaling pathways.Toxicol Appl Pharmacol. 2023 Oct 15;477:116694. doi: 10.1016/j.taap.2023.116694. Epub 2023 Sep 20. Toxicol Appl Pharmacol. 2023. PMID: 37739320 Free PMC article. Review.
Cited by
-
LCZ696, an angiotensin receptor-neprilysin inhibitor, ameliorates diabetic cardiomyopathy by inhibiting inflammation, oxidative stress and apoptosis.Exp Biol Med (Maywood). 2019 Sep;244(12):1028-1039. doi: 10.1177/1535370219861283. Epub 2019 Jul 1. Exp Biol Med (Maywood). 2019. PMID: 31262190 Free PMC article.
-
Distinct Types of Cell Death and the Implication in Diabetic Cardiomyopathy.Front Pharmacol. 2020 Feb 7;11:42. doi: 10.3389/fphar.2020.00042. eCollection 2020. Front Pharmacol. 2020. PMID: 32116717 Free PMC article. Review.
-
A novel GSK3β inhibitor 5n attenuates acute kidney injury.Heliyon. 2024 Apr 12;10(8):e29159. doi: 10.1016/j.heliyon.2024.e29159. eCollection 2024 Apr 30. Heliyon. 2024. PMID: 38644860 Free PMC article.
-
Atorvastatin inhibited TNF-α induced matrix degradation in rat nucleus pulposus cells by suppressing NLRP3 inflammasome activity and inducing autophagy through NF-κB signaling.Cell Cycle. 2021 Oct;20(20):2160-2173. doi: 10.1080/15384101.2021.1973707. Epub 2021 Sep 8. Cell Cycle. 2021. PMID: 34494933 Free PMC article.
-
Andrographolide Ameliorates Diabetic Cardiomyopathy in Mice by Blockage of Oxidative Damage and NF-κB-Mediated Inflammation.Oxid Med Cell Longev. 2018 Jun 25;2018:9086747. doi: 10.1155/2018/9086747. eCollection 2018. Oxid Med Cell Longev. 2018. PMID: 30046380 Free PMC article.
References
-
- Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet. 2011;378(9785):31–40. 10.1016/S0140-6736(11)60679-X - DOI - PubMed
-
- Bertoni AG, Tsai A, Kasper EK, Brancati FL. Diabetes and idiopathic cardiomyopathy: a nationwide case-control study. Diabetes Care. 2003;26(10):2791–2795. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

