Diversification in the HIV-1 Envelope Hyper-variable Domains V2, V4, and V5 and Higher Probability of Transmitted/Founder Envelope Glycosylation Favor the Development of Heterologous Neutralization Breadth
- PMID: 27851829
- PMCID: PMC5112890
- DOI: 10.1371/journal.ppat.1005989
Diversification in the HIV-1 Envelope Hyper-variable Domains V2, V4, and V5 and Higher Probability of Transmitted/Founder Envelope Glycosylation Favor the Development of Heterologous Neutralization Breadth
Abstract
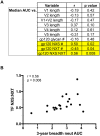
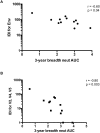
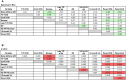
A recent study of plasma neutralization breadth in HIV-1 infected individuals at nine International AIDS Vaccine Initiative (IAVI) sites reported that viral load, HLA-A*03 genotype, and subtype C infection were strongly associated with the development of neutralization breadth. Here, we refine the findings of that study by analyzing the impact of the transmitted/founder (T/F) envelope (Env), early Env diversification, and autologous neutralization on the development of plasma neutralization breadth in 21 participants identified during recent infection at two of those sites: Kigali, Rwanda (n = 9) and Lusaka, Zambia (n = 12). Single-genome analysis of full-length T/F Env sequences revealed that all 21 individuals were infected with a highly homogeneous population of viral variants, which were categorized as subtype C (n = 12), A1 (n = 7), or recombinant AC (n = 2). An extensive amino acid sequence-based analysis of variable loop lengths and glycosylation patterns in the T/F Envs revealed that a lower ratio of NXS to NXT-encoded glycan motifs correlated with neutralization breadth. Further analysis comparing amino acid sequence changes, insertions/deletions, and glycan motif alterations between the T/F Env and autologous early Env variants revealed that extensive diversification focused in the V2, V4, and V5 regions of gp120, accompanied by contemporaneous viral escape, significantly favored the development of breadth. These results suggest that more efficient glycosylation of subtype A and C T/F Envs through fewer NXS-encoded glycan sites is more likely to elicit antibodies that can transition from autologous to heterologous neutralizing activity following exposure to gp120 diversification. This initiates an Env-antibody co-evolution cycle that increases neutralization breadth, and is further augmented over time by additional viral and host factors. These findings suggest that understanding how variation in the efficiency of site-specific glycosylation influences neutralizing antibody elicitation and targeting could advance the design of immunogens aimed at inducing antibodies that can transition from autologous to heterologous neutralizing activity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Limited Evidence for a Relationship between HIV-1 Glycan Shield Features in Early Infection and the Development of Neutralization Breadth.J Virol. 2021 Aug 10;95(17):e0079721. doi: 10.1128/JVI.00797-21. Epub 2021 Aug 10. J Virol. 2021. PMID: 34160251 Free PMC article.
-
Achieving Potent Autologous Neutralizing Antibody Responses against Tier 2 HIV-1 Viruses by Strategic Selection of Envelope Immunogens.J Immunol. 2016 Apr 1;196(7):3064-78. doi: 10.4049/jimmunol.1500527. Epub 2016 Mar 4. J Immunol. 2016. PMID: 26944928 Free PMC article.
-
Conformational Epitope-Specific Broadly Neutralizing Plasma Antibodies Obtained from an HIV-1 Clade C-Infected Elite Neutralizer Mediate Autologous Virus Escape through Mutations in the V1 Loop.J Virol. 2016 Jan 13;90(7):3446-57. doi: 10.1128/JVI.03090-15. J Virol. 2016. PMID: 26763999 Free PMC article.
-
Development of broadly neutralizing antibodies from autologous neutralizing antibody responses in HIV infection.Curr Opin HIV AIDS. 2014 May;9(3):210-6. doi: 10.1097/COH.0000000000000057. Curr Opin HIV AIDS. 2014. PMID: 24662931 Free PMC article. Review.
-
Specificity of the autologous neutralizing antibody response.Curr Opin HIV AIDS. 2009 Sep;4(5):358-63. doi: 10.1097/COH.0b013e32832ea7e8. Curr Opin HIV AIDS. 2009. PMID: 20048698 Free PMC article. Review.
Cited by
-
VH1-69 Utilizing Antibodies Are Capable of Mediating Non-neutralizing Fc-Mediated Effector Functions Against the Transmitted/Founder gp120.Front Immunol. 2019 Jan 15;9:3163. doi: 10.3389/fimmu.2018.03163. eCollection 2018. Front Immunol. 2019. PMID: 30697215 Free PMC article.
-
Neutralization Sensitivity of HIV-1 CRF07_BC From an Untreated Patient With a Focus on Evolution Over Time.Front Cell Infect Microbiol. 2022 Mar 17;12:862754. doi: 10.3389/fcimb.2022.862754. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35372102 Free PMC article.
-
Progress and Challenges in HIV-1 Vaccine Research: A Comprehensive Overview.Vaccines (Basel). 2025 Jan 31;13(2):148. doi: 10.3390/vaccines13020148. Vaccines (Basel). 2025. PMID: 40006695 Free PMC article. Review.
-
High throughput analysis of B cell dynamics and neutralizing antibody development during immunization with a novel clade C HIV-1 envelope.PLoS Pathog. 2023 Oct 25;19(10):e1011717. doi: 10.1371/journal.ppat.1011717. eCollection 2023 Oct. PLoS Pathog. 2023. PMID: 37878666 Free PMC article.
-
Development of broadly neutralizing antibodies in HIV-1 infected elite neutralizers.Retrovirology. 2018 Sep 5;15(1):61. doi: 10.1186/s12977-018-0443-0. Retrovirology. 2018. PMID: 30185183 Free PMC article. Review.
References
-
- Robertson DL, Anderson JP, Bradac JA, Carr JK, Foley B, et al. (2000) HIV-1 nomenclature proposal. Science 288: 55–56. - PubMed
-
- Starcich BR, Hahn BH, Shaw GM, McNeely PD, Modrow S, et al. (1986) Identification and characterization of conserved and variable regions in the envelope gene of HTLV-III/LAV, the retrovirus of AIDS. Cell 45: 637–648. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

