Antimicrobial Peptides Share a Common Interaction Driven by Membrane Line Tension Reduction
- PMID: 27851941
- PMCID: PMC5113125
- DOI: 10.1016/j.bpj.2016.10.003
Antimicrobial Peptides Share a Common Interaction Driven by Membrane Line Tension Reduction
Abstract
Antimicrobial peptides (AMPs) are a class of host-defense molecules that neutralize a broad range of pathogens. Their membrane-permeabilizing behavior has been commonly attributed to the formation of pores; however, with the continuing discovery of AMPs, many are uncharacterized and their exact mechanism remains unknown. Using atomic force microscopy, we previously characterized the disruption of model membranes by protegrin-1 (PG-1), a cationic AMP from pig leukocytes. When incubated with zwitterionic membranes of dimyristoylphosphocholine, PG-1 first induced edge instability at low concentrations, then porous defects at intermediate concentrations, and finally worm-like micelle structures at high concentrations. These rich structural changes suggested that pore formation constitutes only an intermediate state along the route of PG-1's membrane disruption process. The formation of these structures could be best understood by using a mesophase framework of a binary mixture of lipids and peptides, where PG-1 acts as a line-active agent in lowering interfacial bilayer tensions. We have proposed that rather than being static pore formers, AMPs share a common ability to lower interfacial tensions that promote membrane transformations. In a study of 13 different AMPs, we found that peptide line-active behavior was not driven by the overall charge, and instead was correlated with their adoption of imperfect secondary structures. These peptide structures commonly positioned charged residues near the membrane interface to promote deformation favorable for their incorporation into the membrane. Uniquely, the data showed that barrel-stave-forming peptides such as alamethicin are not line-active, and that the seemingly disparate models of toroidal pores and carpet activity are actually related. We speculate that this interplay between peptide structure and the distribution of polar residues in relation to the membrane governs AMP line activity in general and represents a novel, to our knowledge, avenue for the rational design of new drugs.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


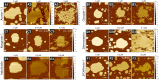
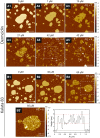
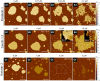
References
-
- Hughes J.M. Preserving the lifesaving power of antimicrobial agents. JAMA. 2011;305:1027–1028. - PubMed
-
- Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. - PubMed
-
- Henderson J.M., Lee K.Y.C. Promising antimicrobial agents designed from natural peptide templates. Curr. Opin. Solid State Mater. Sci. 2013;17:175–192.
-
- Baumann G., Mueller P. A molecular model of membrane excitability. J. Supramol. Struct. 1974;2:538–557. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

