Experimental Estimation of Membrane Tension Induced by Osmotic Pressure
- PMID: 27851942
- PMCID: PMC5112947
- DOI: 10.1016/j.bpj.2016.09.043
Experimental Estimation of Membrane Tension Induced by Osmotic Pressure
Erratum in
-
Experimental Estimation of Membrane Tension Induced by Osmotic Pressure.Biophys J. 2017 Mar 28;112(6):1290. doi: 10.1016/j.bpj.2017.03.001. Biophys J. 2017. PMID: 28355555 Free PMC article. No abstract available.
Abstract
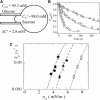
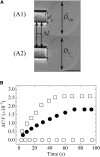
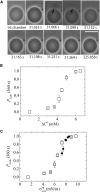
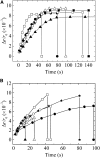
Osmotic pressure (Π) induces the stretching of plasma membranes of cells or lipid membranes of vesicles, which plays various roles in physiological functions. However, there have been no experimental estimations of the membrane tension of vesicles upon exposure to Π. In this report, we estimated experimentally the lateral tension of the membranes of giant unilamellar vesicles (GUVs) when they were transferred into a hypotonic solution. First, we investigated the effect of Π on the rate constant, kp, of constant-tension (σex)-induced rupture of dioleoylphosphatidylcholine (DOPC)-GUVs using the method developed by us recently. We obtained the σex dependence of kp in GUVs under Π and by comparing this result with that in the absence of Π, we estimated the tension of the membrane due to Π at the swelling equilibrium, σosmeq. Next, we measured the volume change of DOPC-GUVs under small Π. The experimentally obtained values of σosmeq and the volume change agreed with their theoretical values within the limits of the experimental errors. Finally, we investigated the characteristics of the Π-induced pore formation in GUVs. The σosmeq corresponding to the threshold Π at which pore formation is induced is similar to the threshold tension of the σex-induced rupture. The time course of the radius change of GUVs in the Π-induced pore formation depends on the total membrane tension, σt; for small σt, the radius increased with time to an equilibrium one, which remained constant for a long time until pore formation, but for large σt, the radius increased with time and pore formation occurred before the swelling equilibrium was reached. Based on these results, we discussed the σosmeq and the Π-induced pore formation in lipid membranes.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
References
-
- Sperelakis N., editor. Cell Physiology Source Book, 4th ed. Essentials of membrane biophysics. Academic press; London: 2012.
-
- Sukharev S.I., Blount P., Kung C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature. 1994;368:265–268. - PubMed
-
- Taupin C., Dvolaitzky M., Sauterey C. Osmotic pressure induced pores in phospholipid vesicles. Biochemistry. 1975;14:4771–4775. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

