Segmentation of 3D Trajectories Acquired by TSUNAMI Microscope: An Application to EGFR Trafficking
- PMID: 27851944
- PMCID: PMC5112935
- DOI: 10.1016/j.bpj.2016.09.041
Segmentation of 3D Trajectories Acquired by TSUNAMI Microscope: An Application to EGFR Trafficking
Abstract
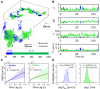
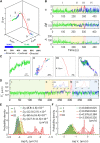
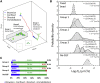
Whereas important discoveries made by single-particle tracking have changed our view of the plasma membrane organization and motor protein dynamics in the past three decades, experimental studies of intracellular processes using single-particle tracking are rather scarce because of the lack of three-dimensional (3D) tracking capacity. In this study we use a newly developed 3D single-particle tracking method termed TSUNAMI (Tracking of Single particles Using Nonlinear And Multiplexed Illumination) to investigate epidermal growth factor receptor (EGFR) trafficking dynamics in live cells at 16/43 nm (xy/z) spatial resolution, with track duration ranging from 2 to 10 min and vertical tracking depth up to tens of microns. To analyze the long 3D trajectories generated by the TSUNAMI microscope, we developed a trajectory analysis algorithm, which reaches 81% segment classification accuracy in control experiments (termed simulated movement experiments). When analyzing 95 EGF-stimulated EGFR trajectories acquired in live skin cancer cells, we find that these trajectories can be separated into three groups-immobilization (24.2%), membrane diffusion only (51.6%), and transport from membrane to cytoplasm (24.2%). When EGFRs are membrane-bound, they show an interchange of Brownian diffusion and confined diffusion. When EGFRs are internalized, transitions from confined diffusion to directed diffusion and from directed diffusion back to confined diffusion are clearly seen. This observation agrees well with the model of clathrin-mediated endocytosis.
Copyright © 2016 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures


References
-
- Shera E.B., Seitzinger N.K., Soper S.A. Detection of single fluorescent molecules. Chem. Phys. Lett. 1990;174:553–557.
-
- Ambrose W.P., Goodwin P.M., Keller R.A. Single molecule fluorescence spectroscopy at ambient temperature. Chem. Rev. 1999;99:2929–2956. - PubMed
-
- Sauer M., Zander C. Single Molecule Detection in Solution. Wiley-VCH Verlag; Hoboken, NJ: 2003. Single molecule identification in solution: Principles and applications; pp. 247–272.
-
- Toomre D., Bewersdorf J. A new wave of cellular imaging. Annu. Rev. Cell Dev. Biol. 2010;26:285–314. - PubMed
-
- Betzig E., Patterson G.H., Hess H.F. Imaging intracellular fluorescent proteins at nanometer resolution. Science. 2006;313:1642–1645. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

