Interim analysis of a phase I/IIa trial assessing E39+GM-CSF, a folate binding protein vaccine, to prevent recurrence in ovarian and endometrial cancer patients
- PMID: 27852036
- PMCID: PMC5362533
- DOI: 10.18632/oncotarget.13305
Interim analysis of a phase I/IIa trial assessing E39+GM-CSF, a folate binding protein vaccine, to prevent recurrence in ovarian and endometrial cancer patients
Abstract
Background: Folate binding protein(FBP) is an immunogenic protein over-expressed in endometrial(EC) and ovarian cancer(OC). We are conducting a phase I/IIa trial of E39 (GALE 301)+GM-CSF, an HLA-A2-restricted, FBP-derived peptide vaccine to prevent recurrences in disease-free EC and OC patients. This interim analysis summarizes toxicity, immunologic responses, and clinical outcomes to date.
Methods: HLA-A2+ patients were vaccinated(VG), and HLA-A2- or -A2+ patients were followed as controls(CG). Six monthly intradermal inoculations of E39+250mcg GM-CSF were administered to VG. Demographic, safety, immunologic, and recurrence rate(RR) data were collected and evaluated.
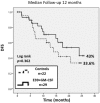
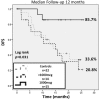
Results: This trial enrolled 51 patients; 29 in the VG and 22 in the CG. Fifteen patients received 1000mcg E39, and 14 received <1000mcg. There were no clinicopathologic differences between groups(all p ≥ 0.1). E39 was well-tolerated regardless of dose. DTH increased pre- to post-vaccination (5.7±1.5 mm vs 10.3±3.0 mm, p = 0.06) in the VG, and increased more in the 1000mcg group (3.8±2.0 mm vs 9.5±3.5 mm, p = 0.03). With 12 months median follow-up, the RR was 41% (VG) vs 55% (CG), p = 0.41. Among the 1000mcg patients, the RR was 13.3% vs 55% CG, p = 0.01. Estimated 2-year DFS was 85.7% in the 1000mcg group vs 33.6% in the CG (p = 0.021).
Conclusions: This phase I/IIa trial reveals that E39+GM-CSF is well-tolerated and elicits a strong, dose-dependent in vivo immune response. Early efficacy results are promising in the 1000 mcg dose cohort. This study proves the safety and establishes the dose of E39 for a larger prospective, randomized, controlled trial in HLA-A2+ EC and OC patients to prevent recurrence.
Keywords: cancer; endometrial; folate binding protein; immunotherapy; ovarian.
Conflict of interest statement
Dr. George E. Peoples, M.D., has inventor rights to the E39 vaccine and serves as a consultant to Galena Biopharma in the development of the vaccine.
The view(s) expressed herein are those of the author(s) and do not reflect the official policy or position of Brooke Army Medical Center, the U.S. Army Medical Department, the U.S. Army Office of the Surgeon General, the Department of the Army, U.S. Air Force Medical Department, the U.S. Air Force Office of the Surgeon General, the Department of the Air Force, Department of Defense or the U.S. Government.
The protocol for this trial was approved by the US Army Medical Research and Material Command's Human Subjects Research Review board, and the individual site's Investigational Review Board. Informed consent was obtained from each patient prior to trial enrollment.
Figures



References
-
- National Comprehensive Cancer Network Guidelines Ovarian Cancer including Fallopian tube Cancer and Primary peritoneal cancer. Version 2.2015.
-
- Markman M, Liu P, Wilczynski S, Monk B, Copeland L, Alvarez R, Jiang C, D; Alberts. Southwest Oncology Group; Gynecologic Oncology Group. Phase III Randomized Trial of 12 versus 3 months of maintenance Paclitaxel in patients with advanced ovarian cancer after complete response to platinum and Paclitaxel-based chemotherapy: A Southwest Oncology Group and Gynecologic Oncology Group Trial. J Clin Oncol. 2003;21:2460–2465. - PubMed
-
- Ziebarth A, Landen C, Jr, Alvarez R. Clinical Obstetrics and Gynecology. Published. Baltimore, MD: 2012. Molecular/Genetic Therapies in Ovarian Cancer: Future Opportunities and Challenges. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

