Clinical validation of prospective liquid biopsy monitoring in patients with wild-type RAS metastatic colorectal cancer treated with FOLFIRI-cetuximab
- PMID: 27852040
- PMCID: PMC5471055
- DOI: 10.18632/oncotarget.13311
Clinical validation of prospective liquid biopsy monitoring in patients with wild-type RAS metastatic colorectal cancer treated with FOLFIRI-cetuximab
Abstract
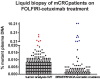
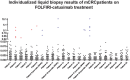
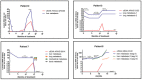
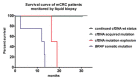
Cancer genomics and translational medicine rely on the molecular profiling of patient's tumor obtained during surgery or biopsy. Alternatively, blood is a less invasive source of tumor DNA shed, amongst other ways, as cell-free DNA (cfDNA). Highly-sensitive assays capable to detect cancer genetic events from patient's blood plasma became popularly known as liquid biopsy (LqB). Importantly, retrospective studies including small number of selected patients with metastatic colorectal cancer (mCRC) patients treated with anti-EGFR therapy have shown LqB capable to detect the acquired clonal mutations in RAS genes leading to therapy resistance. However, the usefulness of LqB in the real-life clinical monitoring of these patients still lack additional validation on controlled studies. In this context, we designed a prospective LqB clinical trial to monitor newly diagnosed KRAS wild-type (wt) mCRC patients who received a standard FOLFIRI-cetuximab regimen. We used BEAMing technique for evaluate cfDNA mutations in KRAS, NRAS, BRAF, and PIK3CA in twenty-five patients during a 2-y period. A total of 2,178 cfDNA mutation analyses were performed and we observed that: a) continued wt circulating status was correlated with a prolonged response; b) smoldering increases in mutant cfDNA were correlated with acquired resistance; while c) mutation upsurge/explosion anticipated a remarkable clinical deterioration. The current study provides evidences, obtained for the first time in an unbiased and prospective manner, that reinforces the utility of LqB for monitoring mCRC patients.
Keywords: anti-EGFR; cetuximab; cfDNA; colorectal cancer; liquid biopsy.
Conflict of interest statement
M Hidalgo has received a consultant's fee from Merck. The other authors disclose no conflicts.
Figures
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics. CA: a cancer journal for clinicians. 2012;62:10–29. - PubMed
-
- Van Cutsem E, Köhne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D'Haens G, Pintér T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. New England Journal of Medicine. 2009;360:1408–17. - PubMed
-
- Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, Simes RJ, Chalchal H, Shapiro JD, Robitaille S, Price TJ, Shepherd L, Au HJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. New England Journal of Medicine. 2008;359:1757–65. - PubMed
-
- Douillard JY1, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, Humblet Y, Bodoky G, Cunningham D, Jassem J, Rivera F, Kocákova I, Ruff P, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. New England Journal of Medicine. 2013;369:1023–34. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

