Therapy-related acute myeloid leukemia following treatment of lymphoid malignancies
- PMID: 27852053
- PMCID: PMC5349887
- DOI: 10.18632/oncotarget.13262
Therapy-related acute myeloid leukemia following treatment of lymphoid malignancies
Abstract
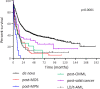
Therapy-related acute myeloid leukemia (t-AML) is a heterogeneous entity most frequently related to breast cancer or lymphoproliferative diseases (LD). Population-based studies have reported an increased risk of t-AML after treatment of lymphomas. The aim of this study was to describe the characteristics and outcome of 80 consecutive cases of t-AML following treatment of LD. t-AML accounted for 2.3% of all AML cases, occurred 60 months after LD diagnosis, and were characterized by a high frequency of FAB M6 AML and poor-risk cytogenetic abnormalities. Time to t-AML diagnosis was influenced by patient age, type of LD, and treatment. Among the 48 t-AML patients treated with intensive chemotherapy, median overall survival (OS) was 7.7 months compared to 26.1 months in de novo, 4.2 months in post-myeloproliferative neoplasm, 9.4 months in post-myelodysplastic syndrome, 8.6 months in post-chronic myelomonocytic leukemia AML, 13.4 months in t-AML secondary to the treatment of solid cancer, and 14.7 months in breast cancer only. OS of post-LD t-AML patients was significantly influenced by age, performance status, myelodysplastic syndrome prior to LD/t-AML, and treatment regimen for LD. Thus, t-AML following lymphoid malignancies treatment should be considered as very high-risk secondary AML. New treatment strategies in patients with LD/t-AML are needed urgently.
Keywords: chronic lymphocytic leukemia; leukemia; lymphoma; second cancer; therapy-related acute myeloid leukemia.
Conflict of interest statement
The authors declare no conflicts of interest related to this study.
Figures
Similar articles
-
Characterization and prognostic features of secondary acute myeloid leukemia in a population-based setting: a report from the Swedish Acute Leukemia Registry.Am J Hematol. 2015 Mar;90(3):208-14. doi: 10.1002/ajh.23908. Epub 2015 Jan 16. Am J Hematol. 2015. PMID: 25421221
-
Is secondary leukemia an independent poor prognostic factor in acute myeloid leukemia?Best Pract Res Clin Haematol. 2007 Mar;20(1):29-37. doi: 10.1016/j.beha.2006.10.006. Best Pract Res Clin Haematol. 2007. PMID: 17336252 Review.
-
A review of therapy-related myelodysplastic syndromes and acute myeloid leukaemia (t-MDS/AML) in Irish patients: a single centre experience.Hematology. 2017 Jul;22(6):341-346. doi: 10.1080/10245332.2017.1286539. Epub 2017 Feb 15. Hematology. 2017. PMID: 28196450
-
Therapy-related myelodysplastic syndrome and acute myeloid leukemia following fludarabine combination chemotherapy.Leukemia. 2010 Dec;24(12):2056-62. doi: 10.1038/leu.2010.218. Epub 2010 Oct 21. Leukemia. 2010. PMID: 20962860
-
Development of acute myeloid leukemia in patients with untreated chronic lymphocytic leukemia.Ann Hematol. 2017 May;96(5):719-724. doi: 10.1007/s00277-017-2933-x. Epub 2017 Feb 1. Ann Hematol. 2017. PMID: 28144729 Review.
Cited by
-
Characteristics and outcomes of patients with lymphoma who developed therapy-related acute myeloid leukemia or myelodysplastic syndrome - a retrospective analysis of the Polish Adult Leukemia Group.Contemp Oncol (Pozn). 2024;28(2):149-157. doi: 10.5114/wo.2024.141727. Epub 2024 Jul 24. Contemp Oncol (Pozn). 2024. PMID: 39421707 Free PMC article.
-
Therapy-related myeloid neoplasms: clinical perspectives.Onco Targets Ther. 2018 Sep 17;11:5909-5915. doi: 10.2147/OTT.S101333. eCollection 2018. Onco Targets Ther. 2018. PMID: 30271175 Free PMC article. Review.
-
Impact of induction chemotherapy with intermediate-dosed cytarabine and subsequent allogeneic stem cell transplantation on the outcome of high-risk acute myeloid leukemia.J Cancer Res Clin Oncol. 2022 Jun;148(6):1481-1492. doi: 10.1007/s00432-021-03733-0. Epub 2021 Jul 23. J Cancer Res Clin Oncol. 2022. PMID: 34297206 Free PMC article.
-
Therapy-related Myeloid Neoplasms: Considerations for Patients' Clinical Evaluation.Mediterr J Hematol Infect Dis. 2023 Sep 1;15(1):e2023051. doi: 10.4084/MJHID.2023.051. eCollection 2023. Mediterr J Hematol Infect Dis. 2023. PMID: 37705524 Free PMC article. Review.
References
-
- Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–302. - PubMed
-
- Swerdlow SH. WHO Classification of tumours of haematopoietic and lymphoid tissues. Lyon, France: IARC; 2008. pp. 109–138.
-
- Mistry AR, Felix CA, Whitmarsh RJ, Mason A, Reiter A, Cassinat B, Parry A, Walz C, Wiemels JL, Segal MR, Adès L, Blair IA, Osheroff N, et al. DNA topoisomerase II in therapy-related acute promyelocytic leukemia. N Engl J Med. 2005;352:1529–38. - PubMed
-
- Kyle RA, Pierre RV, Bayrd ED. Multiple myeloma and acute myelomonocytic leukemia: report of four cases possibly related to melphalan. N Engl J Med. 1970;283:1121–5. - PubMed
-
- Pedersen-Bjergaard J. Insights into leukemogenesis from therapy-related leukemia. N Engl J Med. 2005;352:1591–4. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials