Effects of intravenous iron on fibroblast growth factor 23 (FGF23) in haemodialysis patients: a randomized controlled trial
- PMID: 27852236
- PMCID: PMC5112660
- DOI: 10.1186/s12882-016-0391-7
Effects of intravenous iron on fibroblast growth factor 23 (FGF23) in haemodialysis patients: a randomized controlled trial
Abstract
Background: Intravenous iron affects serum levels of intact fibroblast growth factor-23 (iFGF23) and its cleavage product c-terminal FGF23 (cFGF23) in iron-deficient people with normal renal function. We hypothesized that intravenous iron modulates iFGF23 and cFGF23 in haemodialysis patients differently according to the type of iron used.
Methods: Prevalent, stable haemodialysis patients requiring protocol-based intravenous iron therapy were randomized to a single 200 mg dose of either ferric carboxymaltose (FCM) or iron sucrose (IS). The primary outcome was change in iFGF23 and cFGF23 from pre-infusion to Day 2 post-infusion. Serum hepcidin, ferritin and phosphate were also measured. Pair-wise comparisons utilised the Wilcoxon rank sum test; linear mixed models with an interaction term for treatment and time evaluated between-group effects.
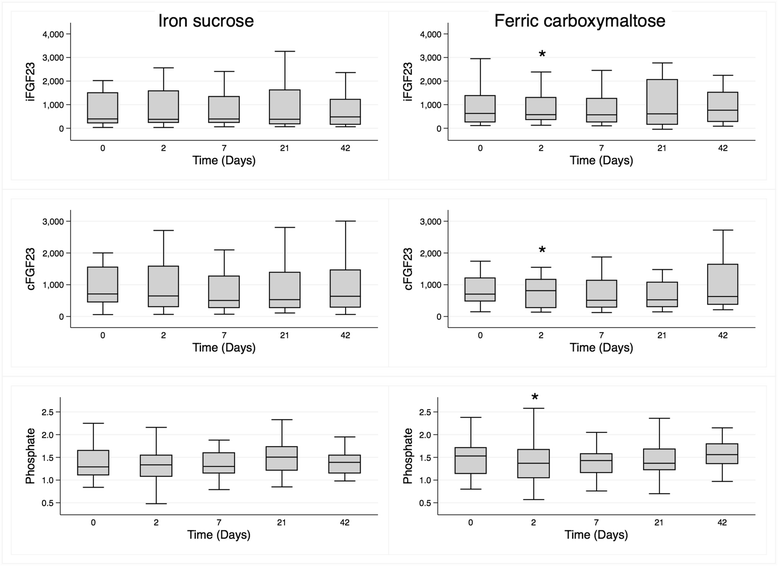
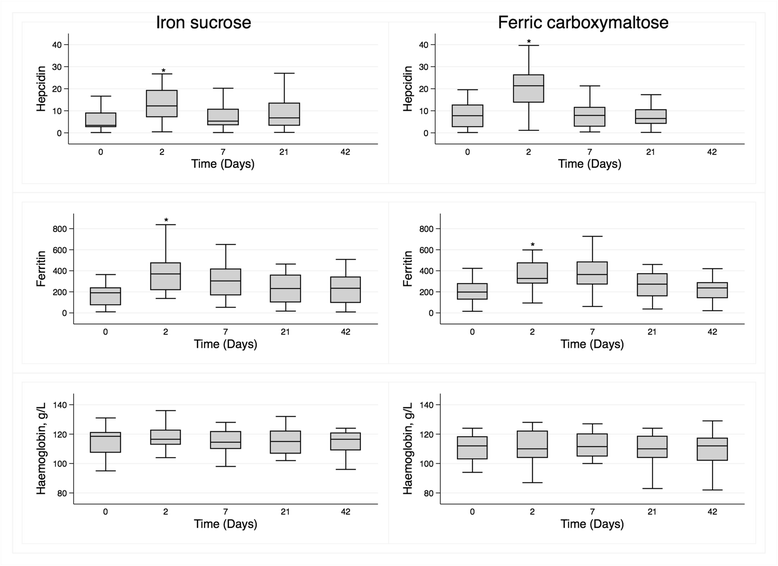
Results: Forty-two participants completed the study. In those randomized to FCM (n = 22), median (interquartile range) values pre-infusion and Day 2, respectively, were 843 pg/mL (313-1922) and 576 pg/mL (356-1296, p = 0.05) for iFGF23, 704RU/mL (475-1204) and 813RU/mL (267-1156, p = 0.04) for cFGF23, and 1.53 mmol/L (1.14-1.71) and 1.37 (1.05-1.67, p = 0.03) for phosphate. These parameters did not change following IS. Both serum ferritin (p < 0.001) and hepcidin (p < 0.001) increased in both groups, and the increase in hepcidin was greater in the FCM group (p = 0.03 for between-group difference).
Conclusions: Contrary to iron-deficient people with normal renal function, haemodialysis patients given protocol-driven intravenous FCM demonstrated a fall in iFGF23 and a rise in cFGF23, changes not evident with IS. This suggests a differential effect of intravenous iron treatment according to both formulation and renal function.
Trial registration: Australian and New Zealand Clinical Trials Register ACTRN12614000548639 . Registered 22 May 2014 (retrospectively registered).
Keywords: Fibroblast growth factor-23; Haemodialysis; Hepcidin; Iron infusion; Randomized controlled trial.
Figures
References
-
- Isakova T, Xie H, Yang W, Xie D, Anderson AH, Scialla J, Wahl P, Gutierrez OM, Steigerwalt S, He J, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;305(23):2432–9. doi: 10.1001/jama.2011.826. - DOI - PMC - PubMed
-
- Farrow EG, Yu X, Summers LJ, Davis SI, Fleet JC, Allen MR, Robling AG, Stayrook KR, Jideonwo V, Magers MJ, et al. Iron deficiency drives an autosomal dominant hypophosphatemic rickets (ADHR) phenotype in fibroblast growth factor-23 (Fgf23) knock-in mice. Proc Natl Acad Sci. 2011;108(46):E1146–55. doi: 10.1073/pnas.1110905108. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

