SPRTN is a mammalian DNA-binding metalloprotease that resolves DNA-protein crosslinks
- PMID: 27852435
- PMCID: PMC5127644
- DOI: 10.7554/eLife.21491
SPRTN is a mammalian DNA-binding metalloprotease that resolves DNA-protein crosslinks
Abstract
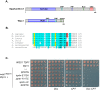
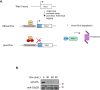
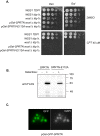
Ruijs-Aalfs syndrome is a segmental progeroid syndrome resulting from mutations in the SPRTN gene. Cells derived from patients with SPRTN mutations elicit genomic instability and people afflicted with this syndrome developed hepatocellular carcinoma. Here we describe the molecular mechanism by which SPRTN contributes to genome stability and normal cellular homeostasis. We show that SPRTN is a DNA-dependent mammalian protease required for resolving cytotoxic DNA-protein crosslinks (DPCs)- a function that had only been attributed to the metalloprotease Wss1 in budding yeast. We provide genetic evidence that SPRTN and Wss1 function distinctly in vivo to resolve DPCs. Upon DNA and ubiquitin binding, SPRTN can elicit proteolytic activity; cleaving DPC substrates and itself. SPRTN null cells or cells derived from patients with Ruijs-Aalfs syndrome are impaired in the resolution of covalent DPCs in vivo. Collectively, SPRTN is a mammalian protease required for resolving DNA-protein crosslinks in vivo whose function is compromised in Ruijs-Aalfs syndrome patients.
Keywords: DNA replication; DNA-protein crosslinks; Dvc1; E. coli; Ruijs-Aalfs syndrome; S. cerevisiae; SPARTAN; SPRTN; Topoisomerase; aging; biochemistry; c1orf124; cell biology; human; metalloprotease; mouse; progeria; ubiquitination.
Conflict of interest statement
ID: Senior editor, eLife. The other authors declare that no competing interests exist.
Figures










References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

