Anemia Offers Stronger Protection Than Sickle Cell Trait Against the Erythrocytic Stage of Falciparum Malaria and This Protection Is Reversed by Iron Supplementation
- PMID: 27852523
- PMCID: PMC5161422
- DOI: 10.1016/j.ebiom.2016.11.011
Anemia Offers Stronger Protection Than Sickle Cell Trait Against the Erythrocytic Stage of Falciparum Malaria and This Protection Is Reversed by Iron Supplementation
Abstract
Background: Iron deficiency causes long-term adverse consequences for children and is the most common nutritional deficiency worldwide. Observational studies suggest that iron deficiency anemia protects against Plasmodium falciparum malaria and several intervention trials have indicated that iron supplementation increases malaria risk through unknown mechanism(s). This poses a major challenge for health policy. We investigated how anemia inhibits blood stage malaria infection and how iron supplementation abrogates this protection.
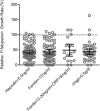
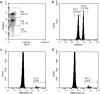
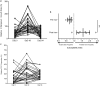
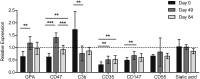
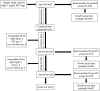
Methods: This observational cohort study occurred in a malaria-endemic region where sickle-cell trait is also common. We studied fresh RBCs from anemic children (135 children; age 6-24months; hemoglobin <11g/dl) participating in an iron supplementation trial (ISRCTN registry, number ISRCTN07210906) in which they received iron (12mg/day) as part of a micronutrient powder for 84days. Children donated RBCs at baseline, Day 49, and Day 84 for use in flow cytometry-based in vitro growth and invasion assays with P. falciparum laboratory and field strains. In vitro parasite growth in subject RBCs was the primary endpoint.
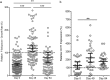
Findings: Anemia substantially reduced the invasion and growth of both laboratory and field strains of P. falciparum in vitro (~10% growth reduction per standard deviation shift in hemoglobin). The population level impact against erythrocytic stage malaria was 15.9% from anemia compared to 3.5% for sickle-cell trait. Parasite growth was 2.4 fold higher after 49days of iron supplementation relative to baseline (p<0.001), paralleling increases in erythropoiesis.
Interpretation: These results confirm and quantify a plausible mechanism by which anemia protects African children against falciparum malaria, an effect that is substantially greater than the protection offered by sickle-cell trait. Iron supplementation completely reversed the observed protection and hence should be accompanied by malaria prophylaxis. Lower hemoglobin levels typically seen in populations of African descent may reflect past genetic selection by malaria.
Funding: National Institute of Child Health and Development, Bill and Melinda Gates Foundation, UK Medical Research Council (MRC) and Department for International Development (DFID) under the MRC/DFID Concordat.
Keywords: Anemia; Hemoglobin; Iron; Iron supplementation; Malaria; Sickle cell trait.
Crown Copyright © 2016. Published by Elsevier B.V. All rights reserved.
Figures

Similar articles
-
Folic acid supplementation and malaria susceptibility and severity among people taking antifolate antimalarial drugs in endemic areas.Cochrane Database Syst Rev. 2022 Feb 1;2(2022):CD014217. doi: 10.1002/14651858.CD014217. Cochrane Database Syst Rev. 2022. PMID: 36321557 Free PMC article.
-
Anemia, Iron Supplementation and Susceptibility to Plasmodium falciparum Malaria.EBioMedicine. 2016 Dec;14:13-14. doi: 10.1016/j.ebiom.2016.11.036. Epub 2016 Dec 1. EBioMedicine. 2016. PMID: 27919755 Free PMC article. No abstract available.
-
Impact of Sickle Cell Trait Hemoglobin on the Intraerythrocytic Transcriptional Program of Plasmodium falciparum.mSphere. 2021 Oct 27;6(5):e0075521. doi: 10.1128/mSphere.00755-21. Epub 2021 Oct 20. mSphere. 2021. PMID: 34668757 Free PMC article.
-
Comparative haematological parameters of HbAA and HbAS genotype children infected with Plasmodium falciparum malaria in Yemen.Hematology. 2014 Apr;19(3):169-74. doi: 10.1179/1607845413Y.0000000113. Epub 2013 Nov 25. Hematology. 2014. PMID: 24074341
-
Iron and malaria: a dangerous liaison?Nutr Rev. 2016 Oct;74(10):612-23. doi: 10.1093/nutrit/nuw021. Epub 2016 Aug 26. Nutr Rev. 2016. PMID: 27566983 Review.
Cited by
-
Common host variation drives malaria parasite fitness in healthy human red cells.Elife. 2021 Sep 23;10:e69808. doi: 10.7554/eLife.69808. Elife. 2021. PMID: 34553687 Free PMC article.
-
Effect of iron fortification on anaemia and risk of malaria among Ghanaian pre-school children with haemoglobinopathies and different ABO blood groups.BMC Nutr. 2023 Mar 23;9(1):56. doi: 10.1186/s40795-023-00709-w. BMC Nutr. 2023. PMID: 36959634 Free PMC article.
-
Multiplication rate variation in the human malaria parasite Plasmodium falciparum.Sci Rep. 2017 Jul 25;7(1):6436. doi: 10.1038/s41598-017-06295-9. Sci Rep. 2017. PMID: 28743888 Free PMC article.
-
Iron Status and Associated Malaria Risk Among African Children.Clin Infect Dis. 2019 May 17;68(11):1807-1814. doi: 10.1093/cid/ciy791. Clin Infect Dis. 2019. PMID: 30219845 Free PMC article.
-
Hepcidin-guided screen-and-treat interventions against iron-deficiency anaemia in pregnancy: a randomised controlled trial in The Gambia.Lancet Glob Health. 2019 Nov;7(11):e1564-e1574. doi: 10.1016/S2214-109X(19)30393-6. Lancet Glob Health. 2019. PMID: 31607468 Free PMC article. Clinical Trial.
References
-
- Brandão M.M., Castro M.d.L.R.B., Fontes A., Cesar C.L., Costa F.F., Saad S.T.O. Impaired red cell deformability in iron deficient subjects. Clin. Hemorheol. Microcirc. 2009;43:217–221. - PubMed
-
- Cox S.E., Doherty C.P., Atkinson S.H., Nweneka C.V., Fulford A.J.C., Sirugo G., Rockett K.A., Kwiatkowski D.P., Prentice A.M. Haptoglobin genotype, anaemia and malaria in Gambian children. Tropical Med. Int. Health. 2008;13:76–82. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

