Rapid Identification of Measles Virus Vaccine Genotype by Real-Time PCR
- PMID: 27852670
- PMCID: PMC5328441
- DOI: 10.1128/JCM.01879-16
Rapid Identification of Measles Virus Vaccine Genotype by Real-Time PCR
Abstract
During measles outbreaks, it is important to be able to rapidly distinguish between measles cases and vaccine reactions to avoid unnecessary outbreak response measures such as case isolation and contact investigations. We have developed a real-time reverse transcription-PCR (RT-PCR) method specific for genotype A measles virus (MeV) (MeVA RT-quantitative PCR [RT-qPCR]) that can identify measles vaccine strains rapidly, with high throughput, and without the need for sequencing to determine the genotype. We have evaluated the method independently in three measles reference laboratories using two platforms, the Roche LightCycler 480 system and the Applied Biosystems (ABI) 7500 real-time PCR system. In comparison to the standard real-time RT-PCR method, the MeVA RT-qPCR showed 99.5% specificity for genotype A and 94% sensitivity for both platforms. The new assay was able to detect RNA from five currently used vaccine strains, AIK-C, CAM-70, Edmonston-Zagreb, Moraten, and Shanghai-191. The MeVA RT-qPCR assay has been used successfully for measles surveillance in reference laboratories, and it could be readily deployed to national and subnational laboratories on a wide scale.
Keywords: PCR; genotyping; measles; measles vaccine; molecular methods.
Copyright © 2017 American Society for Microbiology.
Figures
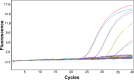
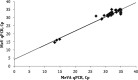
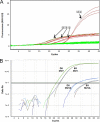

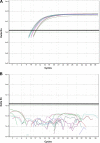
Comment in
-
Progress towards Rapid Detection of Measles Vaccine Strains: a Tool To Inform Public Health Interventions.J Clin Microbiol. 2017 Mar;55(3):686-689. doi: 10.1128/JCM.02329-16. Epub 2016 Dec 21. J Clin Microbiol. 2017. PMID: 28003421 Free PMC article.
Similar articles
-
Laboratory diagnosis of vaccine-associated measles in Zhejiang Province, China.J Microbiol Immunol Infect. 2017 Oct;50(5):578-585. doi: 10.1016/j.jmii.2015.10.004. Epub 2015 Nov 19. J Microbiol Immunol Infect. 2017. PMID: 26698687
-
Utility of a Stressed Single Nucleotide Polymorphism (SNP) Real-Time PCR Assay for Rapid Identification of Measles Vaccine Strains in Patient Samples.J Clin Microbiol. 2018 Jul 26;56(8):e00360-18. doi: 10.1128/JCM.00360-18. Print 2018 Aug. J Clin Microbiol. 2018. PMID: 29743309 Free PMC article.
-
Simultaneous Detection and Differentiation between Wild-Type and Vaccine Measles Viruses by a Multiplex Real-Time Reverse Transcription-PCR Assay.J Clin Microbiol. 2019 Mar 28;57(4):e01828-18. doi: 10.1128/JCM.01828-18. Print 2019 Apr. J Clin Microbiol. 2019. PMID: 30760529 Free PMC article.
-
What assay is optimal for the diagnosis of measles virus infection? An evaluation of the performance of a measles virus real-time reverse transcriptase PCR using the Cepheid SmartCycler(®) and antigen detection by immunofluorescence.J Clin Virol. 2015 Sep;70:46-52. doi: 10.1016/j.jcv.2015.07.004. Epub 2015 Jul 10. J Clin Virol. 2015. PMID: 26305819
-
Detection of measles virus RNA on SYBR green real-time reverse transcription-polymerase chain reaction.Pediatr Int. 2010 Aug;52(4):611-5. doi: 10.1111/j.1442-200X.2010.03124.x. Pediatr Int. 2010. PMID: 20337982
Cited by
-
Characterisation of measles after the introduction of the combined measles-mumps-rubella (MMR) vaccine in 2004 with focus on the laboratory data, 2016 to 2019 outbreak, Romania.Euro Surveill. 2019 Jul;24(29):1900041. doi: 10.2807/1560-7917.ES.2019.24.29.1900041. Euro Surveill. 2019. PMID: 31339098 Free PMC article.
-
Progress towards Rapid Detection of Measles Vaccine Strains: a Tool To Inform Public Health Interventions.J Clin Microbiol. 2017 Mar;55(3):686-689. doi: 10.1128/JCM.02329-16. Epub 2016 Dec 21. J Clin Microbiol. 2017. PMID: 28003421 Free PMC article.
-
Measles Vaccine-Associated Rash Illness in China: an Emerging Issue in the Process of Measles Elimination.J Clin Microbiol. 2020 Oct 21;58(11):e01472-20. doi: 10.1128/JCM.01472-20. Print 2020 Oct 21. J Clin Microbiol. 2020. PMID: 32878947 Free PMC article.
-
Public health responses during measles outbreaks in elimination settings: Strategies and challenges.Hum Vaccin Immunother. 2018;14(9):2222-2238. doi: 10.1080/21645515.2018.1474310. Epub 2018 Jul 11. Hum Vaccin Immunother. 2018. PMID: 29932850 Free PMC article. Review.
-
Recent advances in lab-on-a-chip technologies for viral diagnosis.Biosens Bioelectron. 2020 Apr 1;153:112041. doi: 10.1016/j.bios.2020.112041. Epub 2020 Jan 22. Biosens Bioelectron. 2020. PMID: 31999560 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

