Integrin Cross-Talk Regulates the Human Neutrophil Response to Fungal β-Glucan in the Context of the Extracellular Matrix: A Prominent Role for VLA3 in the Antifungal Response
- PMID: 27852744
- PMCID: PMC5173418
- DOI: 10.4049/jimmunol.1502381
Integrin Cross-Talk Regulates the Human Neutrophil Response to Fungal β-Glucan in the Context of the Extracellular Matrix: A Prominent Role for VLA3 in the Antifungal Response
Abstract
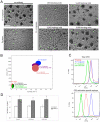
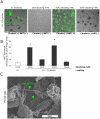
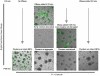
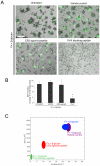
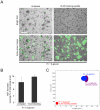
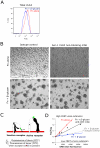
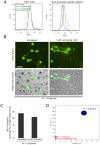
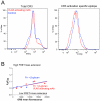
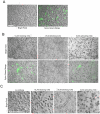
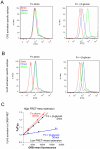
Candida albicans infection produces elongated hyphae resistant to phagocytic clearance compelling alternative neutrophil effector mechanisms to destroy these physically large microbial structures. Additionally, all tissue-based neutrophilic responses to fungal infections necessitate contact with the extracellular matrix (ECM). Neutrophils undergo a rapid, ECM-dependent mechanism of homotypic aggregation and NETosis in response to C. albicans mediated by the β2 integrin, complement receptor 3 (CR3, CD11b/CD18, αMβ2). Neither homotypic aggregation nor NETosis occurs when human neutrophils are exposed either to immobilized fungal β-glucan or to C. albicans hyphae without ECM. The current study provides a mechanistic basis to explain how matrix controls the antifungal effector functions of neutrophils under conditions that preclude phagocytosis. We show that CR3 ligation initiates a complex mechanism of integrin cross-talk resulting in differential regulation of the β1 integrins VLA3 (α3β1) and VLA5 (α5β1). These β1 integrins control distinct antifungal effector functions in response to either fungal β-glucan or C. albicans hyphae and fibronectin, with VLA3 inducing homotypic aggregation and VLA5 regulating NETosis. These integrin-dependent effector functions are controlled temporally whereby VLA5 and CR3 induce rapid, focal NETosis early after binding fibronectin and β-glucan. Within minutes, CR3 undergoes inside-out auto-activation that drives the downregulation of VLA5 and the upregulation of VLA3 to support neutrophil swarming and aggregation. Forcing VLA5 to remain in the activated state permits NETosis but prevents homotypic aggregation. Therefore, CR3 serves as a master regulator during the antifungal neutrophil response, controlling the affinity states of two different β1 integrins, which in turn elicit distinct effector functions.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures

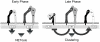
References
-
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin. Infect. Dis. 2004;39:309–317. - PubMed
-
- Marr KA, Carter RA, Crippa F, Wald A, Corey L. Epidemiology and outcome of mould infections in hematopoietic stem cell transplant recipients. Clin. Infect. Dis. 2002;34:909–917. - PubMed
-
- Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal therapy alliance registry. Clin. Infect. Dis. 2009;48:1695–1703. - PubMed
-
- Zaoutis TE, Argon J, Chu J, Berlin JA, Walsh TJ, Feudtner C. The epidemiology and attributable outcomes of candidemia in adults and children hospitalized in the United States: a propensity analysis. Clin. Infect. Dis. 2005;41:1232–1239. - PubMed
-
- Miller LG, Hajjeh RA, Edwards JE., Jr. Estimating the cost of nosocomial candidemia in the united states. Clin. Infect. Dis. 2001;32:1110. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

