Temperature-induced cardiac remodelling in fish
- PMID: 27852752
- PMCID: PMC5278617
- DOI: 10.1242/jeb.128496
Temperature-induced cardiac remodelling in fish
Abstract
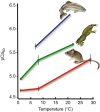
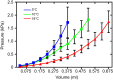
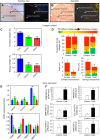
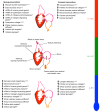
Thermal acclimation causes the heart of some fish species to undergo significant remodelling. This includes changes in electrical activity, energy utilization and structural properties at the gross and molecular level of organization. The purpose of this Review is to summarize the current state of knowledge of temperature-induced structural remodelling in the fish ventricle across different levels of biological organization, and to examine how such changes result in the modification of the functional properties of the heart. The structural remodelling response is thought to be responsible for changes in cardiac stiffness, the Ca2+ sensitivity of force generation and the rate of force generation by the heart. Such changes to both active and passive properties help to compensate for the loss of cardiac function caused by a decrease in physiological temperature. Hence, temperature-induced cardiac remodelling is common in fish that remain active following seasonal decreases in temperature. This Review is organized around the ventricular phases of the cardiac cycle - specifically diastolic filling, isovolumic pressure generation and ejection - so that the consequences of remodelling can be fully described. We also compare the thermal acclimation-associated modifications of the fish ventricle with those seen in the mammalian ventricle in response to cardiac pathologies and exercise. Finally, we consider how the plasticity of the fish heart may be relevant to survival in a climate change context, where seasonal temperature changes could become more extreme and variable.
Keywords: Cardiac function; Cardiac histology; Cardiac remodelling; Connective tissue; Thermal acclimation.
© 2017. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


References
-
- Aho E. and Vornanen M. (1998). Ca2+-ATPase activity and Ca2+ uptake by sarcoplasmic reticulum in fish heart: effects of thermal acclimation. J. Exp. Biol. 201, 525-532. - PubMed
-
- Aho E. and Vornanen M. (1999). Contractile properties of atrial and ventricular myocardium of the heart of rainbow trout Oncorhynchus mykiss: effects of thermal acclimation. J. Exp. Biol. 202, 2663-2677. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

