Activity Induces Fmr1-Sensitive Synaptic Capture of Anterograde Circulating Neuropeptide Vesicles
- PMID: 27852784
- PMCID: PMC5125230
- DOI: 10.1523/JNEUROSCI.2212-16.2016
Activity Induces Fmr1-Sensitive Synaptic Capture of Anterograde Circulating Neuropeptide Vesicles
Abstract
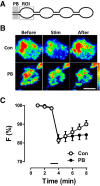
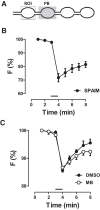
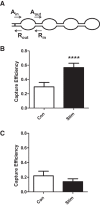
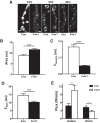
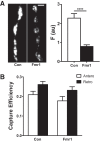
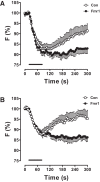
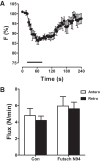
Synaptic neuropeptide and neurotrophin stores are maintained by constitutive bidirectional capture of dense-core vesicles (DCVs) as they circulate in and out of the nerve terminal. Activity increases DCV capture to rapidly replenish synaptic neuropeptide stores following release. However, it is not known whether this is due to enhanced bidirectional capture. Here experiments at the Drosophila neuromuscular junction, where DCVs contain neuropeptides and a bone morphogenic protein, show that activity-dependent replenishment of synaptic neuropeptides following release is evident after inhibiting the retrograde transport with the dynactin disruptor mycalolide B or photobleaching DCVs entering a synaptic bouton by retrograde transport. In contrast, photobleaching anterograde transport vesicles entering a bouton inhibits neuropeptide replenishment after activity. Furthermore, tracking of individual DCVs moving through boutons shows that activity selectively increases capture of DCVs undergoing anterograde transport. Finally, upregulating fragile X mental retardation 1 protein (Fmr1, also called FMRP) acts independently of futsch/MAP-1B to abolish activity-dependent, but not constitutive, capture. Fmr1 also reduces presynaptic neuropeptide stores without affecting activity-independent delivery and evoked release. Therefore, presynaptic motoneuron neuropeptide storage is increased by a vesicle capture mechanism that is distinguished from constitutive bidirectional capture by activity dependence, anterograde selectivity, and Fmr1 sensitivity. These results show that activity recruits a separate mechanism than used at rest to stimulate additional synaptic capture of DCVs for future release of neuropeptides and neurotrophins.
Significance statement: Synaptic release of neuropeptides and neurotrophins depends on presynaptic accumulation of dense-core vesicles (DCVs). At rest, DCVs are captured bidirectionally as they circulate through Drosophila motoneuron terminals by anterograde and retrograde transport. Here we show that activity stimulates further synaptic capture that is distinguished from basal capture by its selectivity for anterograde DCVs and its inhibition by overexpression of the fragile X retardation protein Fmr1. Fmr1 dramatically lowers DCV numbers in synaptic boutons. Therefore, activity-dependent anterograde capture is a major determinant of presynaptic peptide stores.
Keywords: SPAIM; axonal transport; peptidergic transmission; secretory granule; synaptic capture.
Copyright © 2016 the authors 0270-6474/16/3611781-07$15.00/0.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
