Phosphorylation of Human Retinoid X Receptor α at Serine 260 Impairs Its Subcellular Localization, Receptor Interaction, Nuclear Mobility, and 1α,25-Dihydroxyvitamin D3-dependent DNA Binding in Ras-transformed Keratinocytes
- PMID: 27852823
- PMCID: PMC5270490
- DOI: 10.1074/jbc.M116.758185
Phosphorylation of Human Retinoid X Receptor α at Serine 260 Impairs Its Subcellular Localization, Receptor Interaction, Nuclear Mobility, and 1α,25-Dihydroxyvitamin D3-dependent DNA Binding in Ras-transformed Keratinocytes
Abstract
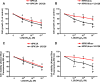
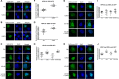
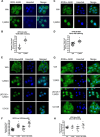
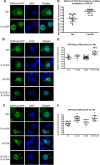
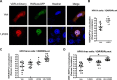
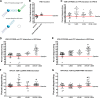
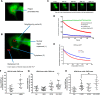
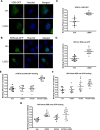
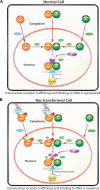
Human retinoid X receptor α (hRXRα) plays a critical role in DNA binding and transcriptional activity through heterodimeric association with several members of the nuclear receptor superfamily, including the human vitamin D receptor (hVDR). We previously showed that hRXRα phosphorylation at serine 260 through the Ras-Raf-MAPK ERK1/2 activation is responsible for resistance to the growth inhibitory effects of 1α,25-dihydroxyvitamin D3 (1α,25(OH)2D3), the biologically active metabolite of vitamin D3 To further investigate the mechanism of this resistance, we studied intranuclear dynamics of hVDR and hRXRα-tagged constructs in living cells together with endogenous and tagged protein in fixed cells. We find that hVDR-, hRXRα-, and hVDR-hRXRα complex accumulate in the nucleus in 1α,25(OH)2D3-treated HPK1A cells but to a lesser extent in HPK1ARas-treated cells. Also, by using fluorescence resonance energy transfer (FRET), we demonstrate increased interaction of the hVDR-hRXRα complex in 1α,25(OH)2D3-treated HPK1A but not HPK1ARas cells. In HPK1ARas cells, 1α,25(OH)2D3-induced nuclear localization and interaction of hRXRα are restored when cells are treated with the MEK1/2 inhibitor UO126 or following transfection of the non-phosphorylatable hRXRα Ala-260 mutant. Finally, we demonstrate using fluorescence loss in photobleaching and quantitative co-localization with chromatin that RXR immobilization and co-localization with chromatin are significantly increased in 1α,25(OH)2D3-treated HPK1ARas cells transfected with the non-phosphorylatable hRXRα Ala-260 mutant. This suggests that hRXRα phosphorylation significantly disrupts its nuclear localization, interaction with VDR, intra-nuclear trafficking, and binding to chromatin of the hVDR-hRXR complex.
Keywords: fluorescence loss in photobleaching (FLIP); fluorescence resonance energy transfer (FRET); mitogen-activated protein kinase (MAPK); phosphorylation; retinoid; retinoid X receptor; vitamin D; vitamin D receptor.
© 2017 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures
Similar articles
-
Examination of VDR/RXR/DRIP205 Interaction, Intranuclear Localization, and DNA Binding in Ras-Transformed Keratinocytes and Its Implication for Designing Optimal Vitamin D Therapy in Cancer.Endocrinology. 2018 Mar 1;159(3):1303-1327. doi: 10.1210/en.2017-03098. Endocrinology. 2018. PMID: 29300860
-
Phosphorylation of the human retinoid X receptor alpha at serine 260 impairs coactivator(s) recruitment and induces hormone resistance to multiple ligands.J Biol Chem. 2008 Feb 22;283(8):4943-56. doi: 10.1074/jbc.M707517200. Epub 2007 Nov 14. J Biol Chem. 2008. PMID: 18003614
-
Mitogen-activated protein kinase inhibits 1,25-dihydroxyvitamin D3-dependent signal transduction by phosphorylating human retinoid X receptor alpha.J Clin Invest. 1999 Jun;103(12):1729-35. doi: 10.1172/JCI6871. J Clin Invest. 1999. PMID: 10377179 Free PMC article.
-
The vitamin D hormone and its nuclear receptor: molecular actions and disease states.J Endocrinol. 1997 Sep;154 Suppl:S57-73. J Endocrinol. 1997. PMID: 9379138 Review.
-
Current understanding of the function of the nuclear vitamin D receptor in response to its natural and synthetic ligands.Recent Results Cancer Res. 2003;164:29-42. doi: 10.1007/978-3-642-55580-0_2. Recent Results Cancer Res. 2003. PMID: 12899512 Review.
Cited by
-
Triclosan treatment decreased the antitumor effect of sorafenib on hepatocellular carcinoma cells.Onco Targets Ther. 2018 May 18;11:2945-2954. doi: 10.2147/OTT.S165436. eCollection 2018. Onco Targets Ther. 2018. PMID: 29849464 Free PMC article.
-
The Impact of Vitamin D and L-Cysteine Co-Supplementation on Upregulating Glutathione and Vitamin D-Metabolizing Genes and in the Treatment of Circulating 25-Hydroxy Vitamin D Deficiency.Nutrients. 2024 Jun 24;16(13):2004. doi: 10.3390/nu16132004. Nutrients. 2024. PMID: 38999752 Free PMC article. Review.
-
Mitogen-Activated Protein Kinase and Nuclear Hormone Receptor Crosstalk in Cancer Immunotherapy.Int J Mol Sci. 2023 Sep 4;24(17):13661. doi: 10.3390/ijms241713661. Int J Mol Sci. 2023. PMID: 37686465 Free PMC article. Review.
-
9q34 & 16p13 chromosome duplications in autism.AME Case Rep. 2020 Jul 30;4:17. doi: 10.21037/acr.2020.03.07. eCollection 2020. AME Case Rep. 2020. PMID: 32793859 Free PMC article.
-
The Interactions of Insulin and Vitamin A Signaling Systems for the Regulation of Hepatic Glucose and Lipid Metabolism.Cells. 2021 Aug 21;10(8):2160. doi: 10.3390/cells10082160. Cells. 2021. PMID: 34440929 Free PMC article. Review.
References
-
- van Leeuwen J. P., van Driel M., van den Bemd G. J., and Pols H. A. (2001) Vitamin D control of osteoblast function and bone extracellular matrix mineralization. Crit. Rev. Eukaryot. Gene Expr. 11, 199–226 - PubMed
-
- Chen J., Dosier C. R., Park J. H., De S., Guldberg R. E., Boyan B. D., and Schwartz Z. (2016) Mineralization of three-dimensional osteoblast cultures is enhanced by the interaction of 1α,25-dihydroxyvitamin D3 and BMP2 via two specific vitamin D receptors. J. Tissue Eng. Regen. Med. 10, 40–51 - PubMed
-
- Haussler M. R., Whitfield G. K., Kaneko I., Haussler C. A., Hsieh D., Hsieh J. C., and Jurutka P. W. (2013) Molecular mechanisms of vitamin D action. Calcif. Tissue Int. 92, 77–98 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

