Differential Receptor Binding and Regulatory Mechanisms for the Lymphangiogenic Growth Factors Vascular Endothelial Growth Factor (VEGF)-C and -D
- PMID: 27852824
- PMCID: PMC5207153
- DOI: 10.1074/jbc.M116.736801
Differential Receptor Binding and Regulatory Mechanisms for the Lymphangiogenic Growth Factors Vascular Endothelial Growth Factor (VEGF)-C and -D
Abstract
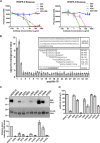
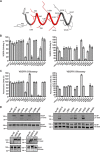
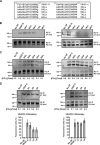
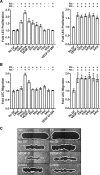
VEGF-C and VEGF-D are secreted glycoproteins that induce angiogenesis and lymphangiogenesis in cancer, thereby promoting tumor growth and spread. They exhibit structural homology and activate VEGFR-2 and VEGFR-3, receptors on endothelial cells that signal for growth of blood vessels and lymphatics. VEGF-C and VEGF-D were thought to exhibit similar bioactivities, yet recent studies indicated distinct signaling mechanisms (e.g. tumor-derived VEGF-C promoted expression of the prostaglandin biosynthetic enzyme COX-2 in lymphatics, a response thought to facilitate metastasis via the lymphatic vasculature, whereas VEGF-D did not). Here we explore the basis of the distinct bioactivities of VEGF-D using a neutralizing antibody, peptide mapping, and mutagenesis to demonstrate that the N-terminal α-helix of mature VEGF-D (Phe93-Arg108) is critical for binding VEGFR-2 and VEGFR-3. Importantly, the N-terminal part of this α-helix, from Phe93 to Thr98, is required for binding VEGFR-3 but not VEGFR-2. Surprisingly, the corresponding part of the α-helix in mature VEGF-C did not influence binding to either VEGFR-2 or VEGFR-3, indicating distinct determinants of receptor binding by these growth factors. A variant of mature VEGF-D harboring a mutation in the N-terminal α-helix, D103A, exhibited enhanced potency for activating VEGFR-3, was able to promote increased COX-2 mRNA levels in lymphatic endothelial cells, and had enhanced capacity to induce lymphatic sprouting in vivo This mutant may be useful for developing protein-based therapeutics to drive lymphangiogenesis in clinical settings, such as lymphedema. Our studies shed light on the VEGF-D structure/function relationship and provide a basis for understanding functional differences compared with VEGF-C.
Keywords: angiogenesis; endothelial cell; lymphangiogenesis; mutagenesis in vitro; receptor; vascular endothelial growth factor (VEGF).
© 2016 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures

Similar articles
-
Structural determinants of vascular endothelial growth factor-D receptor binding and specificity.Blood. 2011 Feb 3;117(5):1507-15. doi: 10.1182/blood-2010-08-301549. Epub 2010 Dec 8. Blood. 2011. PMID: 21148085
-
Molecular control of lymphatic metastasis.Ann N Y Acad Sci. 2008;1131:225-34. doi: 10.1196/annals.1413.020. Ann N Y Acad Sci. 2008. PMID: 18519975 Review.
-
The tyrosine kinase inhibitor cediranib blocks ligand-induced vascular endothelial growth factor receptor-3 activity and lymphangiogenesis.Cancer Res. 2008 Jun 15;68(12):4754-62. doi: 10.1158/0008-5472.CAN-07-5809. Cancer Res. 2008. PMID: 18559522
-
Lymphangiogenesis in aortic valve stenosis--novel regulatory roles for valvular myofibroblasts and mast cells.Atherosclerosis. 2012 Apr;221(2):366-74. doi: 10.1016/j.atherosclerosis.2011.12.034. Epub 2011 Dec 29. Atherosclerosis. 2012. PMID: 22281299
-
Molecular targeting of lymphatics for therapy.Curr Pharm Des. 2004;10(1):65-74. doi: 10.2174/1381612043453513. Curr Pharm Des. 2004. PMID: 14754406 Review.
Cited by
-
Potential lymphangiogenesis therapies: Learning from current antiangiogenesis therapies-A review.Med Res Rev. 2018 Sep;38(6):1769-1798. doi: 10.1002/med.21496. Epub 2018 Mar 12. Med Res Rev. 2018. PMID: 29528507 Free PMC article. Review.
-
The Efficacy and Safety of Continuous Intravenous Endostar Treatment Combined With Concurrent Chemoradiotherapy in Patients With Locally Advanced Cervical Squamous Cell Carcinoma: A Randomized Controlled Trial.Front Oncol. 2021 Aug 13;11:723193. doi: 10.3389/fonc.2021.723193. eCollection 2021. Front Oncol. 2021. PMID: 34485157 Free PMC article.
-
Current Concepts and Methods in Tissue Interface Scaffold Fabrication.Biomimetics (Basel). 2022 Oct 4;7(4):151. doi: 10.3390/biomimetics7040151. Biomimetics (Basel). 2022. PMID: 36278708 Free PMC article. Review.
-
Engineered biomaterial strategies for controlling growth factors in tissue engineering.Drug Deliv. 2020 Dec;27(1):1438-1451. doi: 10.1080/10717544.2020.1831104. Drug Deliv. 2020. PMID: 33100031 Free PMC article. Review.
-
Application of microscale culture technologies for studying lymphatic vessel biology.Microcirculation. 2019 Nov;26(8):e12547. doi: 10.1111/micc.12547. Epub 2019 May 2. Microcirculation. 2019. PMID: 30946511 Free PMC article. Review.
References
-
- Stacker S. A., Williams S. P., Karnezis T., Shayan R., Fox S. B., and Achen M. G. (2014) Lymphangiogenesis and lymphatic vessel remodelling in cancer. Nat. Rev. Cancer 14, 159–172 - PubMed
-
- Duong T., Koltowska K., Pichol-Thievend C., Le Guen L., Fontaine F., Smith K. A., Truong V., Skoczylas R., Stacker S. A., Achen M. G., Koopman P., Hogan B. M., and Francois M. (2014) VEGFD regulates blood vascular development by modulating SOX18 activity. Blood 123, 1102–1112 - PubMed
-
- Karnezis T., Shayan R., Caesar C., Roufail S., Harris N. C., Ardipradja K., Zhang Y. F., Williams S. P., Farnsworth R. H., Chai M. G., Rupasinghe T. W., Tull D. L., Baldwin M. E., Sloan E. K., Fox S. B., Achen M. G., and Stacker S. A. (2012) VEGF-D promotes tumor metastasis by regulating prostaglandins produced by the collecting lymphatic endothelium. Cancer Cell 21, 181–195 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

