Age-Dependent Differences in Pseudorabies Virus Neuropathogenesis and Associated Cytokine Expression
- PMID: 27852848
- PMCID: PMC5215323
- DOI: 10.1128/JVI.02058-16
Age-Dependent Differences in Pseudorabies Virus Neuropathogenesis and Associated Cytokine Expression
Abstract
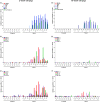
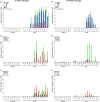
The severity of clinical symptoms induced by pseudorabies virus (PRV) infection of its natural host is inversely related to the age of the pig. During this study, 2- and 15-week-old pigs were inoculated with PRV strain NIA3. This resulted in important clinical disease, although the associated morbidity and mortality were lower in older pigs. Quantitative PCR analysis of viral DNA in different organs confirmed the general knowledge on PRV pathogenesis. Several new findings and potential explanations for the observed age-dependent differences in virulence, however, were determined from the study of viral and cytokine mRNA expression at important sites of neuropathogenesis. First, only limited viral and cytokine mRNA expression was detected in the nasal mucosa, suggesting that other sites may serve as the primary replication site. Second, PRV reached the trigeminal ganglion (TG) and brain stem rapidly upon infection but, compared to 2-week-old pigs, viral replication was less pronounced in 15-week-old pigs, and the decrease in viral mRNA expression was not preceded by or associated with an increased cytokine expression. Third, extensive viral replication associated with a robust expression of cytokine mRNA was detected in the olfactory bulbs of pigs from both age categories and correlated with the observed neurological disease. Our results suggest that age-dependent differences in PRV-induced clinical signs are probably due to enhanced viral replication and associated immunopathology in immature TG and the central nervous system neurons of 2-week-old pigs and that neurological disease is related with extensive viral replication and an associated immune response in the olfactory bulb.
Importance: It is well known that alphaherpesvirus infections of humans and animals result in more severe clinical disease in newborns than in older individuals and that this is probably related to differences in neuropathogenesis. The underlying mechanisms, however, remain unclear. Pseudorabies virus infection of its natural host, the pig, provides a suitable infection model to study this more profoundly. We show here that the severe neurological disease observed in 2-week-old pigs does not appear to be related to a hampered innate immune response but is more likely to reflect the immature development state of the trigeminal ganglia (TG) and central nervous system (CNS) neurons, resulting in an inefficient suppression of viral replication. In 15-week-old pigs, viral replication was efficiently suppressed in the TG and CNS without induction of an extensive immune response. Furthermore, our results provide evidence that neurological disease could, at least in part, be related to viral replication and associated immunopathology in the olfactory bulb.
Keywords: neuropathogenesis; olfactory bulb; pseudorabies virus; trigeminal ganglion; virulence.
Copyright © 2017 American Society for Microbiology.
Figures




Similar articles
-
Reduced virulence of a pseudorabies virus isolate from wild boar origin in domestic pigs correlates with hampered visceral spread and age-dependent reduced neuroinvasive capacity.Virulence. 2018 Jan 1;9(1):149-162. doi: 10.1080/21505594.2017.1368941. Epub 2017 Oct 4. Virulence. 2018. PMID: 28873002 Free PMC article.
-
Glycoprotein gE-negative pseudorabies virus has a reduced capability to infect second- and third-order neurons of the olfactory and trigeminal routes in the porcine central nervous system.J Gen Virol. 1994 Nov;75 ( Pt 11):3095-106. doi: 10.1099/0022-1317-75-11-3095. J Gen Virol. 1994. PMID: 7964619
-
Glycoprotein D-negative pseudorabies virus can spread transneuronally via direct neuron-to-neuron transmission in its natural host, the pig, but not after additional inactivation of gE or gI.J Virol. 1996 Apr;70(4):2191-200. doi: 10.1128/JVI.70.4.2191-2200.1996. J Virol. 1996. PMID: 8642642 Free PMC article.
-
Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine.Microbiol Mol Biol Rev. 2005 Sep;69(3):462-500. doi: 10.1128/MMBR.69.3.462-500.2005. Microbiol Mol Biol Rev. 2005. PMID: 16148307 Free PMC article. Review.
-
Pseudorabies virus infections in pigs. Role of viral proteins in virulence, pathogenesis and transmission.Vet Res. 1997;28(1):1-17. Vet Res. 1997. PMID: 9172836 Review.
Cited by
-
Pseudorabies gD protein protects mice and piglets against lethal doses of pseudorabies virus.Front Microbiol. 2023 Nov 6;14:1288458. doi: 10.3389/fmicb.2023.1288458. eCollection 2023. Front Microbiol. 2023. PMID: 38029147 Free PMC article.
-
Age-dependent differences in type I interferon, IL-12 and pro-inflammatory cytokine production by porcine peripheral blood mononuclear cells in response to pseudorabies virus-infected cells.Front Immunol. 2025 Jul 3;16:1596490. doi: 10.3389/fimmu.2025.1596490. eCollection 2025. Front Immunol. 2025. PMID: 40677723 Free PMC article.
-
Weaning causes a prolonged but transient change in immune gene expression in the intestine of piglets.J Anim Sci. 2021 Apr 1;99(4):skab065. doi: 10.1093/jas/skab065. J Anim Sci. 2021. PMID: 33640983 Free PMC article.
-
Efficient control of Japanese encephalitis virus in the central nervous system of infected pigs occurs in the absence of a pronounced inflammatory immune response.J Neuroinflammation. 2020 Oct 23;17(1):315. doi: 10.1186/s12974-020-01974-3. J Neuroinflammation. 2020. PMID: 33097065 Free PMC article.
-
Alphaherpesvirus infection of mice primes PNS neurons to an inflammatory state regulated by TLR2 and type I IFN signaling.PLoS Pathog. 2019 Nov 1;15(11):e1008087. doi: 10.1371/journal.ppat.1008087. eCollection 2019 Nov. PLoS Pathog. 2019. PMID: 31675371 Free PMC article.
References
-
- Pol JMA, Gielkens ALJ, Oirschot Van JT. 1989. Comparative pathogenesis of three strains of pseudorabies virus in pigs. Microbial 361–371. - PubMed
-
- Sabó A, Rajcáni J, Blasković D. 1968. Studies on the pathogenesis of Aujeszky's disease. I. Distribution of the virulent virus in piglets after peroral infection. Acta Virol 12:214–221. - PubMed
-
- Sabó A, Rajcáni J, Blaskovic D. 1969. Studies on the pathogenesis of Aujeszky's disease. III. The distribution of virulent virus in piglets after intranasal infection. Acta Virol 13:407–414. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

