Robust Central Nervous System Pathology in Transgenic Mice following Peripheral Injection of α-Synuclein Fibrils
- PMID: 27852849
- PMCID: PMC5215322
- DOI: 10.1128/JVI.02095-16
Robust Central Nervous System Pathology in Transgenic Mice following Peripheral Injection of α-Synuclein Fibrils
Abstract
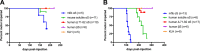
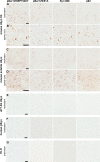
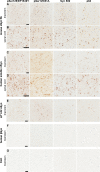
Misfolded α-synuclein (αS) is hypothesized to spread throughout the central nervous system (CNS) by neuronal connectivity leading to widespread pathology. Increasing evidence indicates that it also has the potential to invade the CNS via peripheral nerves in a prion-like manner. On the basis of the effectiveness following peripheral routes of prion administration, we extend our previous studies of CNS neuroinvasion in M83 αS transgenic mice following hind limb muscle (intramuscular [i.m.]) injection of αS fibrils by comparing various peripheral sites of inoculations with different αS protein preparations. Following intravenous injection in the tail veins of homozygous M83 transgenic (M83+/+) mice, robust αS pathology was observed in the CNS without the development of motor impairments within the time frame examined. Intraperitoneal (i.p.) injections of αS fibrils in hemizygous M83 transgenic (M83+/-) mice resulted in CNS αS pathology associated with paralysis. Interestingly, injection with soluble, nonaggregated αS resulted in paralysis and pathology in only a subset of mice, whereas soluble Δ71-82 αS, human βS, and keyhole limpet hemocyanin (KLH) control proteins induced no symptoms or pathology. Intraperitoneal injection of αS fibrils also induced CNS αS pathology in another αS transgenic mouse line (M20), albeit less robustly in these mice. In comparison, i.m. injection of αS fibrils was more efficient in inducing CNS αS pathology in M83 mice than i.p. or tail vein injections. Furthermore, i.m. injection of soluble, nonaggregated αS in M83+/- mice also induced paralysis and CNS αS pathology, although less efficiently. These results further demonstrate the prion-like characteristics of αS and reveal its efficiency to invade the CNS via multiple routes of peripheral administration.
Importance: The misfolding and accumulation of α-synuclein (αS) inclusions are found in a number of neurodegenerative disorders and is a hallmark feature of Parkinson's disease (PD) and PD-related diseases. Similar characteristics have been observed between the infectious prion protein and αS, including its ability to spread from the peripheral nervous system and along neuroanatomical tracts within the central nervous system. In this study, we extend our previous results and investigate the efficiency of intravenous (i.v.), intraperitoneal (i.p.), and intramuscular (i.m.) routes of injection of αS fibrils and other protein controls. Our data reveal that injection of αS fibrils via these peripheral routes in αS-overexpressing mice are capable of inducing a robust αS pathology and in some cases cause paralysis. Furthermore, soluble, nonaggregated αS also induced αS pathology, albeit with much less efficiency. These findings further support and extend the idea of αS neuroinvasion from peripheral exposures.
Keywords: Lewy pathology; alpha-synuclein; peripheral; prions; transgenic mice.
Copyright © 2017 American Society for Microbiology.
Figures



References
-
- Goedert M. 1997. Familial Parkinson's disease. The awakening of alpha-synuclein. Nature 388:232–233. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

