Identification of Human Anti-HIV gp160 Monoclonal Antibodies That Make Effective Immunotoxins
- PMID: 27852851
- PMCID: PMC5244328
- DOI: 10.1128/JVI.01955-16
Identification of Human Anti-HIV gp160 Monoclonal Antibodies That Make Effective Immunotoxins
Abstract
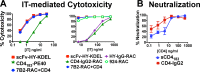
The envelope (Env) glycoprotein of HIV is the only intact viral protein expressed on the surface of both virions and infected cells. Env is the target of neutralizing antibodies (Abs) and has been the subject of intense study in efforts to produce HIV vaccines. Therapeutic anti-Env Abs can also exert antiviral effects via Fc-mediated effector mechanisms or as cytotoxic immunoconjugates, such as immunotoxins (ITs). In the course of screening monoclonal antibodies (MAbs) for their ability to deliver cytotoxic agents to infected or Env-transfected cells, we noted disparities in their functional activities. Different MAbs showed diverse functions that did not correlate with each other. For example, MAbs against the external loop region of gp41 made the most effective ITs against infected cells but did not neutralize virus and bound only moderately to the same cells that they killed so effectively when they were used in ITs. There were also differences in IT-mediated killing among transfected and infected cell lines that were unrelated to the binding of the MAb to the target cells. Our studies of a well-characterized antigen demonstrate that MAbs against different epitopes have different functional activities and that the binding of one MAb can influence the interaction of other MAbs that bind elsewhere on the antigen. These results have implications for the use of MAbs and ITs to kill HIV-infected cells and eradicate persistent reservoirs of HIV infection.
Importance: There is increased interest in using antibodies to treat and cure HIV infection. Antibodies can neutralize free virus and kill cells already carrying the virus. The virus envelope (Env) is the only HIV protein expressed on the surfaces of virions and infected cells. In this study, we examined a panel of human anti-Env antibodies for their ability to deliver cell-killing toxins to HIV-infected cells and to perform other antiviral functions. The ability of an antibody to make an effective immunotoxin could not be predicted from its other functional characteristics, such as its neutralizing activity. Anti-HIV immunotoxins could be used to eliminate virus reservoirs that persist despite effective antiretroviral therapy.
Keywords: HIV envelope; epitope; gp160; immunotoxin; monoclonal antibody.
Copyright © 2017 American Society for Microbiology.
Figures



Similar articles
-
Design and In Vivo Characterization of Immunoconjugates Targeting HIV gp160.J Virol. 2017 Jan 18;91(3):e01360-16. doi: 10.1128/JVI.01360-16. Print 2017 Feb 1. J Virol. 2017. PMID: 27795412 Free PMC article.
-
In vitro effects of anti-HIV immunotoxins directed against multiple epitopes on HIV type 1 envelope glycoprotein 160.AIDS Res Hum Retroviruses. 1996 Jul 20;12(11):1041-51. doi: 10.1089/aid.1996.12.1041. AIDS Res Hum Retroviruses. 1996. PMID: 8827220
-
Across Functional Boundaries: Making Nonneutralizing Antibodies To Neutralize HIV-1 and Mediate Fc-Mediated Effector Killing of Infected Cells.mBio. 2021 Oct 26;12(5):e0140521. doi: 10.1128/mBio.01405-21. Epub 2021 Sep 28. mBio. 2021. PMID: 34579568 Free PMC article.
-
Envelope-specific antibodies and antibody-derived molecules for treating and curing HIV infection.Nat Rev Drug Discov. 2016 Dec;15(12):823-834. doi: 10.1038/nrd.2016.173. Epub 2016 Oct 7. Nat Rev Drug Discov. 2016. PMID: 27725635 Free PMC article. Review.
-
Antibodies for Human Immunodeficiency Virus-1 Cure Strategies.J Infect Dis. 2021 Feb 15;223(12 Suppl 2):22-31. doi: 10.1093/infdis/jiaa165. J Infect Dis. 2021. PMID: 33586772 Free PMC article. Review.
Cited by
-
Photoinduced Photosensitizer-Antibody Conjugates Kill HIV Env-Expressing Cells, Also Inactivating HIV.ACS Omega. 2021 Jun 8;6(25):16524-16534. doi: 10.1021/acsomega.1c01721. eCollection 2021 Jun 29. ACS Omega. 2021. PMID: 34235324 Free PMC article.
-
Photoimmunotherapy Using Cationic and Anionic Photosensitizer-Antibody Conjugates against HIV Env-Expressing Cells.Int J Mol Sci. 2020 Dec 1;21(23):9151. doi: 10.3390/ijms21239151. Int J Mol Sci. 2020. PMID: 33271741 Free PMC article.
-
Identification of Anti-gp41 Monoclonal Antibodies That Effectively Target Cytotoxic Immunoconjugates to Cells Infected with Human Immunodeficiency Virus, Type 1.Vaccines (Basel). 2023 Apr 12;11(4):829. doi: 10.3390/vaccines11040829. Vaccines (Basel). 2023. PMID: 37112741 Free PMC article.
-
A Toxin-Conjugated Recombinant Protein Targeting gp120 and gp41 for Inactivating HIV-1 Virions and Killing Latency-Reversing Agent-Reactivated Latent Cells.mBio. 2022 Feb 22;13(1):e0338421. doi: 10.1128/mbio.03384-21. Epub 2022 Jan 18. mBio. 2022. PMID: 35038908 Free PMC article.
-
Targeted Nanocarrier Delivery of RNA Therapeutics to Control HIV Infection.Pharmaceutics. 2022 Jun 26;14(7):1352. doi: 10.3390/pharmaceutics14071352. Pharmaceutics. 2022. PMID: 35890248 Free PMC article. Review.
References
-
- Zhou T, Georgiev I, Wu X, Yang Z-Y, Dai K, Finzi A, Do Kwon Y, Scheid J, Shi W, Xu L, Yang Y, Zhu J, Nussenzweig MC, Sodroski J, Shapiro L, Nabel GJ, Mascola JR, Kwong PD. 2010. Structural basis for broad and potent neutralization of HIV-1 by antibody VRC01. Science 329:811–817. doi:10.1126/science.1192819. - DOI - PMC - PubMed
-
- Walker LM, Phogat SK, Chan-Hui P-Y, Wagner D, Phung P, Goss JL, Wrin T, Simek MD, Fling S, Mitcham JL, Lehrman JK, Priddy FH, Olsen OA, Frey SM, Hammond PW, Protocol G Principal Investigators, Kaminsky S, Zamb T, Moyle M, Koff WC, Poignard P, Burton DR. 2009. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science 326:285–289. doi:10.1126/science.1178746. - DOI - PMC - PubMed
-
- Walker LM, Huber M, Doores KJ, Falkowska E, Pejchal R, Julien J-P, Wang S-K, Ramos A, Chan-Hui P-Y, Moyle M, Mitcham JL, Hammond PW, Olsen OA, Phung P, Fling S, Wong C-H, Phogat S, Wrin T, Simek MD, Protocol G Principal Investigators, Koff WC, Wilson IA, Burton DR, Poignard P. 2011. Broad neutralization coverage of HIV by multiple highly potent antibodies. Nature 477:466–470. doi:10.1038/nature10373. - DOI - PMC - PubMed
-
- Huang J, Ofek G, Laub L, Louder MK, Doria-Rose NA, Longo NS, Imamichi H, Bailer RT, Chakrabarti B, Sharma SK, Alam SM, Wang T, Yang Y, Zhang B, Migueles SA, Wyatt R, Haynes BF, Kwong PD, Mascola JR, Connors M. 2012. Broad and potent neutralization of HIV-1 by a gp41-specific human antibody. Nature 491:406–412. doi:10.1038/nature11544. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

