Kaposi's Sarcoma-Associated Herpesvirus MicroRNAs Target GADD45B To Protect Infected Cells from Cell Cycle Arrest and Apoptosis
- PMID: 27852859
- PMCID: PMC5244352
- DOI: 10.1128/JVI.02045-16
Kaposi's Sarcoma-Associated Herpesvirus MicroRNAs Target GADD45B To Protect Infected Cells from Cell Cycle Arrest and Apoptosis
Abstract
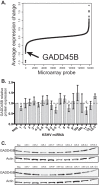
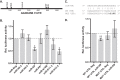
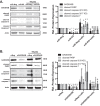
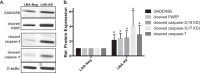
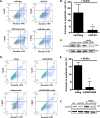
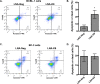
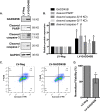
Kaposi's sarcoma is one of the most common malignancies in HIV-infected individuals. The responsible agent, Kaposi's sarcoma-associated herpesvirus (KSHV; HHV8), expresses multiple microRNAs (miRNAs), but the targets and functions of these miRNAs are not completely understood. After infection in primary endothelial cells with KSHV, growth arrest DNA damage-inducible gene 45 beta (GADD45B) is one of the most repressed genes using genomic expression profiling. GADD45B was also repressed in mRNA expression profiling experiments when KSHV miRNAs were introduced to uninfected cells. We hypothesized that KSHV miRNAs target human GADD45B to protect cells from consequences of DNA damage, which can be triggered by viral infection. Expression of GADD45B protein is induced by the p53 activator, Nutlin-3, and KSHV miRNA-K9 inhibits this induction. In addition, Nutlin-3 increased apoptosis and cell cycle arrest based on flow cytometry assays. KSHV miR-K9 protected primary endothelial cells from apoptosis and cell cycle arrest following Nutlin-3 treatment. Similar protective phenotypes were seen for targeting GADD45B with short interfering RNAs (siRNAs), as with miR-K9. KSHV miR-K9 also decreased the protein levels of cleaved caspase-3, cleaved caspase-7, and cleaved poly(ADP-ribose) polymerase (PARP). In B lymphocytes latently infected with KSHV, specific inhibitors of KSHV miR-K9 led to increased GADD45B expression and apoptosis, indicating that miR-K9 is important for reducing apoptosis in infected cells. Furthermore, ectopic expression of GADD45B in KSHV-infected cells promoted apoptosis. Together, these results identify a new miRNA target and demonstrate that KSHV miRNAs are important for protecting infected cells from DNA damage responses.
Importance: Kaposi's sarcoma-associated herpesvirus is a leading cause of cancers in individuals with AIDS. Promoting survival of infected cells is essential for maintaining viral infections. A virus needs to combat various cellular defense mechanisms designed to eradicate the viral infection. One such response can include DNA damage response factors, which can promote an arrest in cell growth and trigger cell death. We used a new approach to search for human genes repressed by small nucleic acids (microRNAs) expressed by a gammaherpesvirus (KSHV), which identified a gene called GADD45B as a target of microRNAs. Repression of GADD45B, which is expressed in response to DNA damage, benefited survival of infected cells in response to a DNA damage response. This information could be used to design new treatments for herpesvirus infections.
Keywords: DNA damage; Kaposi's sarcoma-associated herpesvirus; cell cycle; microRNA.
Copyright © 2017 American Society for Microbiology.
Figures

References
-
- Gottwein E, Corcoran DL, Mukherjee N, Skalsky RL, Hafner M, Nusbaum JD, Shamulailatpam P, Love CL, Dave SS, Tuschl T, Ohler U, Cullen BR. 2011. Viral microRNA targetome of KSHV-infected primary effusion lymphoma cell lines. Cell Host Microbe 10:515–526. doi: 10.1016/j.chom.2011.09.012. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

